Visual Abstract
Abstract
Decreasing reserves of fossil fuels and environmental concerns like greenhouse gases have brought increasing studies into renewable and low-carbon sources of energy. Biodiesel is a renewable and sustainable replacement for crude oil that has become increasingly important as the world’s supply of petroleum decreases, and it is vitally important to research new feedstocks with high efficiency, competitive pricing, and ease of manufacture. Herein we reported nine different biodiesels that were produced from the nine common vegetable oils coconut oil, sesame oil, poppy oil, castor oil, bitter almond oil, olive oil, black seed oil, peanut oil, and pecan oil. The yields of the produced biodiesels are in the range of 50 to 98%, and the density and flashpoints of the produced biodiesels were in the ranges of 0.85 to 0.99 (g/cm3) and 150 to 222°C. Moreover, compositions of the produced biodiesels were analyzed by GC, and the concentrations of the main fatty acid methyl esters (FAME) were reported and compared. Lauric acid methyl ester, oleic acid methyl ester, linoleic acid methyl ester, ricinoleic acid methyl ester, and stearic acid methyl ester are the main components of the produced biodiesels.
Introduction
Different energy resources are used all over the world, but among them, fossil fuels are the vital importance to any country. The resources of fossil fuels are limited and therefore investigation of the renewable alternative fuels is an important and increasing need for all countries. Biodiesel is one of the renewable energy sources, which consists of mono-alkyl esters of long-chain fatty acids [1] and has many advantages such as having reduced greenhouse emissions [2] fuel properties close to petroleum diesel [3], being non-toxic, environmentally safe and biodegradable [4].
Transesterification is the most common way for the production of biodiesel [5,6]. In this reaction, the oil is reacted with an alcohol in the presence of a suitable catalyst such as acid or alkaline catalysts [7]. Ethanol and methanol are the most common alcohols used for the production of biodiesel, because of their low cost and proper reaction rate [8]. By comparing different catalysts, alkaline catalysts such as sodium and potassium hydroxide are more effective in the transesterification reaction [9] and they were used as catalysts of the reaction for the production of biodiesel.
When food-grade vegetable oils like cottonseed oil or soybean oil are used as the feedstock for biodiesel production, the total cost of produced biodiesel is relatively high and the raw material costs consist of about 70–95% of the total cost of biodiesel product [10]. Hence, by using non-food feedstocks such as waste cooking oil, micro-algae, poppy oil, bitter almond oil, or animal fats, the production cost is reduced efficiently to about 90–70% [11].
Recently, the main countries which were produced biodiesel on a large scale, selected production technology routes that align with their respective natural resources. For example, Argentina, Brazil, and the United States utilize soybean oil as the raw material for biodiesel production, while rapeseed oil is extremely used by the European governments and China predominantly relies on waste oil [12].
Many edible and non-edible oils were used as the feedstock for the biodiesel production process [13]. Among them, non-edible oils are the lower-cost feedstocks, and usually, the non-edible oil crops are well-grown on wastelands in dry climate conditions and do not need intensive care [14].
In this work, nine edible and non-edible vegetable oils were selected as the feedstock and they were used in transesterification reaction for biodiesel production. Then, the yield and some other characteristics of the produced biodiesels were compared and the main produced fatty acid methyl esters were reported. The selected feedstocks are extremely extracted from the native plants of Iran. Previously, some of these feedstocks have been studied by other researchers in different experimental conditions [15–18]. However, the environmental conditions of the cultivation place may have a great impact on the compositions of the oil feedstocks, and as a result, they may produce different biodiesels with different compositions. Moreover, based on our knowledge, all of these feedstocks have not been studied and compared at the same time. In the present work, the transesterification of different feedstocks was conducted in the same experimental conditions and the results were reported.
Materials and Methods
Materials
The used chemicals of sodium hydroxide (NaOH) (purity 99 %) and methanol (CH3OH) (99.9 % purity), were purchased from Ghatran-shimi (Tehran, Iran). The nine different vegetable oils of coconut oil, Sesame oil, Poppy oil, Castor oil, Bitter almond oil, olive oil, Black seed oil, Peanut oil, and Pecan oil were prepared from the supermarket. Double distilled water and acetone were used for dilution and washing.
Biodiesel production method
The transesterification process was performed in a 250 ml conical flask, which is connected to a reflux condenser, and to maintain the desired temperature, the system was immersed in a glycerin bath. For heating and agitation, a hot plate with magnetic stirring is used. The constant conditions of 65°C, atmospheric pressure, 1-hour reaction time, and mixing rate of 500 rpm were used for the transesterification reaction.
For the transesterification process, the methanol-catalyst solution was prepared by mixing 0.2 g of NaOH catalyst with 60 ml methanol for 15 min. Then, 10 ml of vegetable oil was heated to 65°C and the methanol-catalyst solution was added to it and mixed for 60 min at 65°C. Then, the mixture was decanted for 24 h (Fig. 1), and the upper phase was separated. The produced biodiesel was washed three times with distilled water and it remained for 60 min in a rotary evaporator at 80°C for separation of any remaining water or alcohol. Finally, the excess methanol was recovered by distillation.

The yield of produced biodiesel
To reduce production costs, the efficiency of produced biodiesel is an important parameter for the selection of feedstock, type of catalyst, and other effective parameters.
For calculation of the yields of produced biodiesels, the biodiesels produced in the previous section were washed, purified, and weighed. Finally, the efficiencies of biodiesels produced with the nine different oil feedstocks were calculated by equation (1).
\(\text{The yield of produced biodiesel}=\frac{\text{The weight of produced biodiesel}}{\text{the weight of oil feestocks}}\times 100\)
Biodiesel characterization methods
A glass pycnometer with a volume of 5 ml was used to measure the oil density. ASTM D 92 method was used as a closed container method to measure the flash point of the produced biodiesel.
The chemical compositions of the produced biodiesels were analyzed by the GC method. The produced biodiesels were first distilled and the obtained products were injected into a gas chromatography machine.
A Cp–3800 Varian gas chromatography device was equipped with a 30-meter capillary column with an internal diameter of 0.25 mm and an FID detector. The detector and injector temperatures were constant and equal to 180 and 200°C respectively. Argon gas with a flow rate of 1 ml/min was used as the carrier gas. The split ratio was 1:5 and the injection volume was 1 µlit. The oven temperature of the device was set at 60°C, which was increased after a 3-minute break at a rate of 5°C/min to the target temperature of 180°C.
Base Oil | Major FAME in the produced biodiesel (Wt%) | |||
Coconut oil | Lauric acid M.E. (59.13 %) | Myristic acid M.E. (5.69 %) | Palmitic acid M.E. (6.49 %) | Oleic acid M.E. (5.1 %) |
Sesame oil | Stearic acid M.E. (5.67 %) | Oleic acid M.E. (36.46 %) | Linoleic acid M.E. (36.95 %) | Lignoceric acid M.E. (12.36 %) |
Poppy oil | Linoleic acid M.E. (19.54%) | Oleic acid M.E. (39.96%) | Palmitic acid M.E. (14.70%) | Stearic acid M.E. (6.04%) |
Castor oil | Oleic acid M.E. (6.24%) | Ricinoleic acid M.E. (39.098%) | Linoleic acid M.E. (14.18%) | Saturated fatty acids (18.48%) |
Bitter almond oil | Linoleic acid M.E. (41.21 %) | Oleic acid M.E. (10.12 %) | Palmitic acid M.E. (6.81 %) |
|
A mixture of sesame and olive oil | Stearic acid M.E. (5.61%) | Oleic acid M.E. (56.63 %) | Linoleic acid M.E. (20.17 %) | Lignoceric acid M.E. (5.55 %) |
Black seed oil | Linoleic acid M.E. (26.8 %) | Oleic acid M.E. (16.5 %) | Palmitic acid M.E. (16.1 %) | Miristic acid M.E. (19.1%) |
Peanut oil | Palmitic acid M.E. (2.87 %) | Linoleic acid M.E. (21.02 %) | Oleic acid M.E. (22.0 %) | Arachidic acid M.E. (3.65 %) |
Pecan oil | Linoleic acid M.E. (21.36 % ) | Palmitic acid M.E. 14.2% (31.78) | Miristic acid M.E. (11.30 %) | Stearic acid M.E. (20.42 %) |
Results and Discussion
Comparison between the yields, densities, and flash points of the produced biodiesels
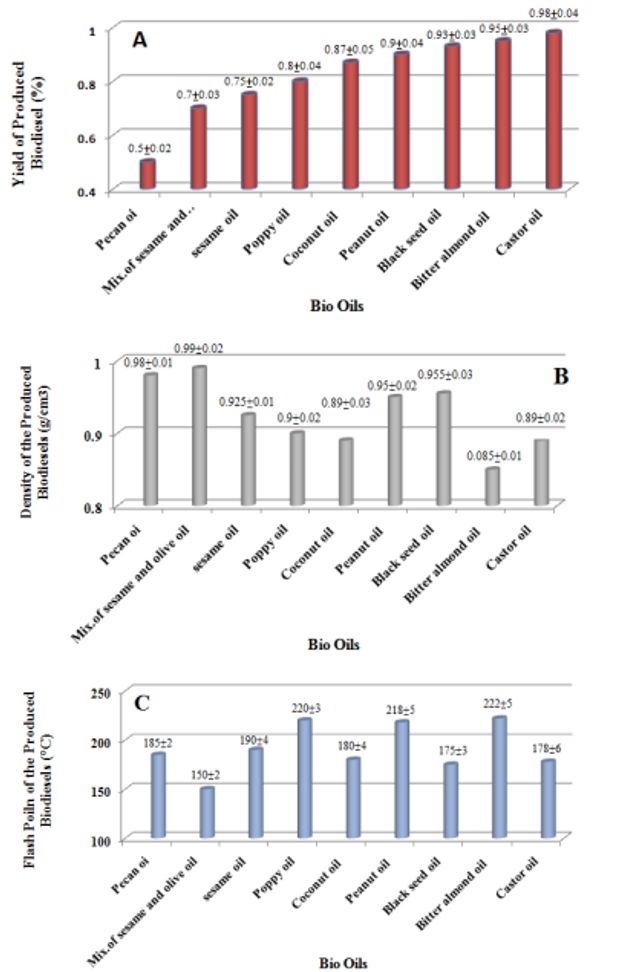
In this study, a similar procedure was used to produce biodiesel from the nine vegetable oil samples. The yields of produced biodiesels by using the nine different oil feedstocks are shown in Fig. 2 (a). As can be seen from the results of this figure, castor oil, bitter almond oil, and black seed oil have the highest production yields, and on the contrary, bitter almond oil has the lowest biodiesel production yield. The reported values for the yields of produced biodiesels are relatively in agreement with the results of the literature specifically for black seed oil [15], castor oil [16], and coconut oil [17].
Fig. 2 (b) shows the density of the produced biodiesels by using the nine different oil feedstocks. The densities of the biodiesels produced from different oils are between 0.985 and 0.85 g/cm3. Biodiesel produced from bitter almond oil has the lowest density and the mixture of sesame and olive oil has the highest density.
According to the values reported in the ASTM D6751, the biodiesels produced from poppy, sesame, coconut, and castor oils are found to fulfill ASTM D6751 [21] (related to the density of the biodiesel).
The values of flash points for the biodiesels produced by using the nine different oil feedstocks are shown in Fig. 2 (c). The value of the flash point in a fuel sample is related to high vapor pressure components that exist in the sample. The range of flash points of the produced biodiesels varies between 150 and 216°C. Biodiesel produced from poppy, bitter almond, and peanut oils has the highest flash point, while biodiesel produced from sesame oil has a flash point within the ASTM D6751 standard range.
Comparison between the main components of the produced biodiesels
In this section, the chemical compositions of feedstocks and the composition of the produced biodiesels after the transesterification reaction have been investigated. This study was done by gas chromatography method and the type and concentration of different methyl esters produced from the fatty acids in the primary oils were detected.
Table 1 shows the most important esters in the biodiesels produced from different oils along with their percentage concentrations. As can be seen, Oleic acid M. E., Linoleic acid M. E., Lauric acid M. E., Palmitic acid M. E., Stearic acid M. E., and Myristic acid M. E. are the main chemical components in the produced biodiesels. These values are in agreement with the reported results in the literature [3,5,15,16,17,18]. In the rest of this section, we will examine the gas chromatogram peaks obtained from some samples.
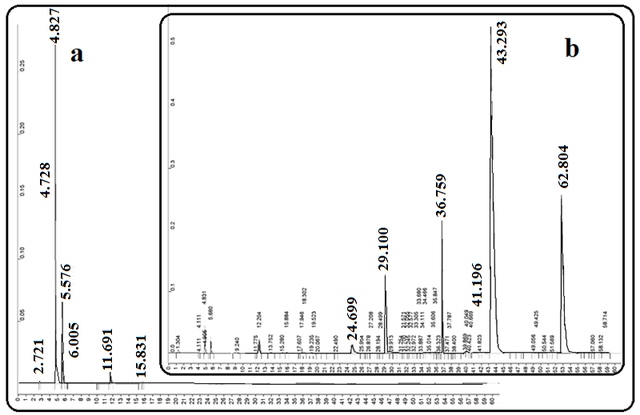
Fig. 3 shows the GC chromatograms of coconut oil and the biodiesels produced after the transesterification of coconut oil. As can be seen in this figure, the transesterification process on the fatty acids that exist in coconut oil produces the corresponding methyl esters. Among the most important substances in biodiesel obtained from coconut oil, we can mention the esters of lauric acid methyl ester, myristic acid methyl ester, and palmitic acid methyl ester with a total concentration of about 70 %.
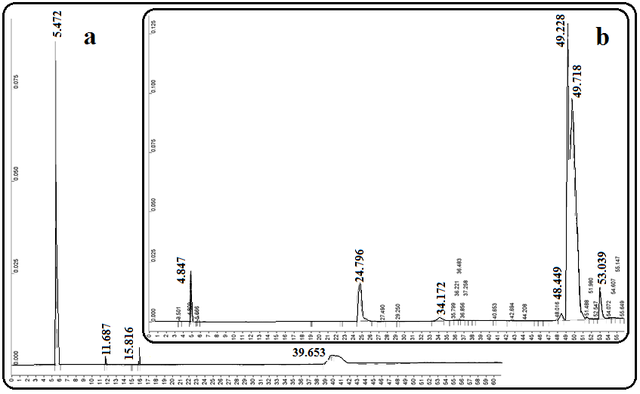
The GC chromatograms of the primary sesame oil and the biodiesel produced after the transesterification process are shown in Fig. 4. Linoleic acid methyl ester, oleic acid methyl ester, stearic acid methyl ester, and palmitic acid methyl ester are the most important esters of fatty acids in this biodiesel sample, and their total concentrations in the produced biodiesel are about 80%.
Conclusions
The experimental findings about the production of biodiesel as a renewable source of energy from the nine different edible and non-edible vegetable oils were reported in this work. Based on the reported results, castor oil and bitter almond oil are converted to biodiesels with maximum yields of higher than 95%. Biodiesels produced from the feedstocks of poppy oil, sesame oil, coconut oil, and castor oil have densities between 0.89–0.92 (g/cm3) which are fulfilled with the ASTM D6751 density range. However, biodiesels produced from castor oil, black seed oil, coconut oil, and pecan oil have the flash point values fulfilled with ASTM D6751 (175–185°C). Therefore, the produced biodiesels have relatively good characteristics for industrial uses. Moreover, Oleic and linoleic acid methyl esters are the most important components in relatively all the produced biodiesels. However, lauric acid methyl ester and ricinoleic acid methyl ester have the highest compositions in biodiesels produced from coconut oil and castor oil feedstocks.
Statements and Declarations
Authors' contributions
All authors have the same contribution.
Competing interests
The authors declare no competing interests.
Ethics Approval
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
No funding was received to support this work.
Authors Information
Hadi Baseri—School of Chemistry, Damghan University, Damghan, Iran.
Niloufar Ghaani—School of Chemistry, Damghan University, Damghan, Iran.



.jpg)
 - Copy copy.png)


