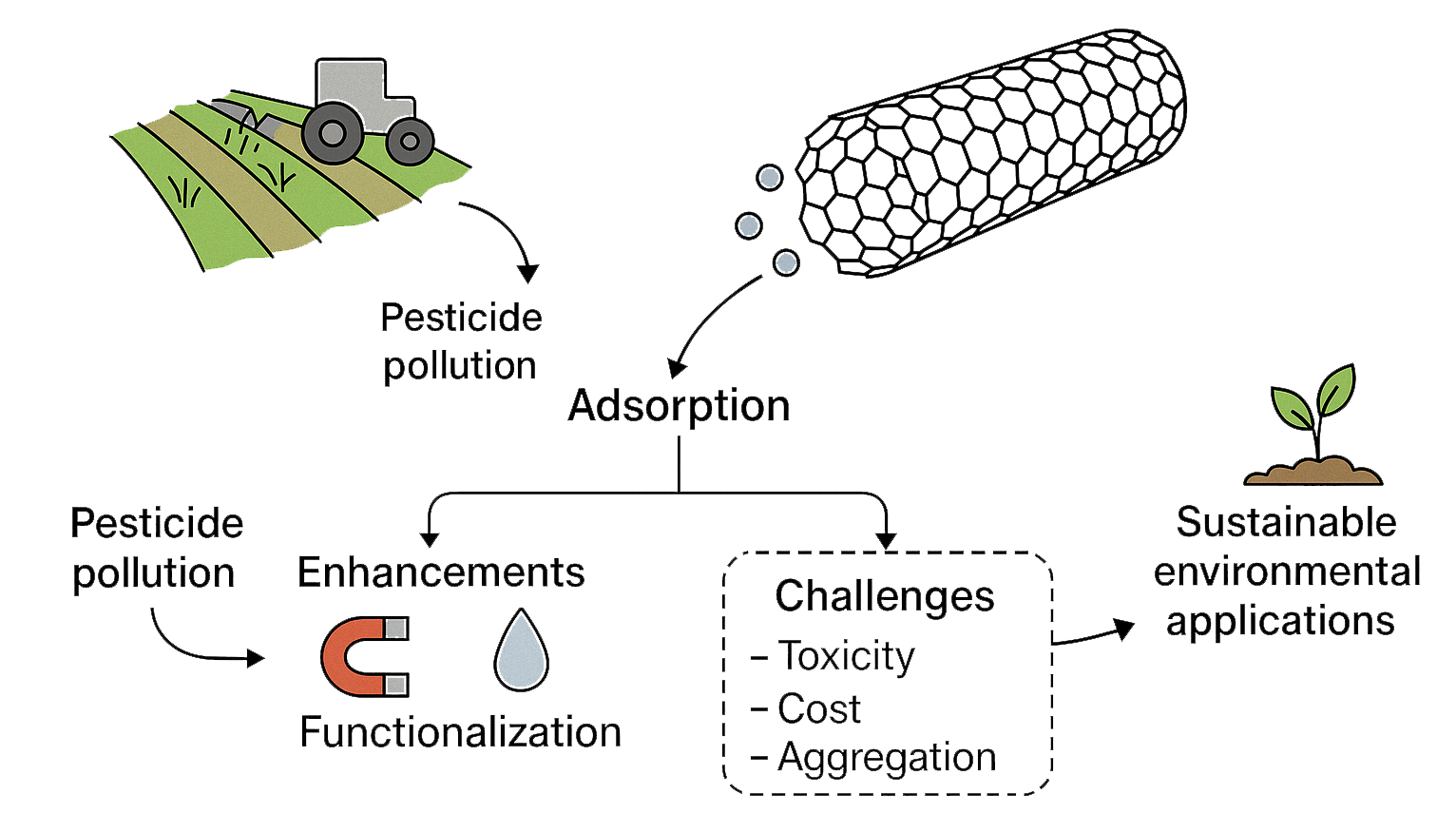
Visual Abstract
Abstract
Pesticides are vital for protecting crops, but their overuse has led to serious environmental and health concerns. Carbon nanotubes (CNTs) have emerged as a promising tool for removing these pollutants due to their large surface area, strong adsorption capacity, and unique structural properties. Compared to activated carbon and graphene oxide, CNTs exhibit higher adsorption capacities and faster kinetics for pesticide removal due to their larger surface area, stronger π–π interactions, and tunable surface chemistry. This review highlights how CNTs—particularly when functionalized or combined with other materials—can effectively capture and detect pesticide residues. It also explores recent advances, challenges like toxicity and cost, and the need for safer, more sustainable applications. As research progresses, CNTs could play a key role in cleaner, more responsible agriculture.
Carbon nanotubes (CNTs), Pesticide adsorption, Functionalized CNTs, CNT-Based sensors
Introduction
Pesticides play an indispensable role in modern agriculture, significantly contributing to global food security by mitigating crop losses caused by pests and diseases. These chemical agents encompass a broad range of compounds designed to control or eliminate weeds (herbicides), insects (insecticides), fungi (fungicides), and other pests that threaten agricultural productivity. However, while the benefits of pesticides in ensuring high crop yields are undeniable, their excessive and unregulated use poses substantial environmental and human health risks. Pesticide contamination of various ecological compartments—such as air, soil, and water—has become a pressing global concern due to its detrimental effects on non-target organisms and overall ecosystem stability. A comprehensive study in the Marmara Basin, Türkiye’s most densely populated and agriculturally intensive region, identified the use of 173 pesticide active substances (ASs). Among these, 52 ASs were classified as high-risk based on usage amounts, hazardous properties, and environmental monitoring results. Notably, pesticides such as cypermethrin and aclonifen exceeded environmental quality standards in various water bodies within the basin, underscoring the urgent need for improved monitoring and regulation of pesticide application [1,2,3,4]
Among the primary environmental concerns associated with pesticide usage is water contamination. Pesticides enter aquatic ecosystems through multiple pathways, including surface runoff, leaching, spray drift, and subsurface drainage. Once in water bodies, these compounds accumulate and persist, leading to widespread contamination of rivers, lakes, groundwater, and even drinking water sources [5]. The persistence of pesticide residues in water not only affects aquatic biodiversity but also poses significant risks to human health, potentially leading to chronic toxicity, endocrine disruption, neurological disorders, and carcinogenic effects [6].
To mitigate pesticide contamination in the environment, various remediation techniques have been explored, among which solid-phase extraction (SPE) has emerged as one of the most effective and widely utilized approaches. SPE operates through adsorption mechanisms that involve both physical and chemical interactions between the sorbent material and the pesticide molecules. Physical adsorption relies on weak intermolecular forces such as Van der Waals interactions, whereas chemical adsorption involves stronger interactions such as covalent bonding or electron exchange. While chemical adsorption ensures a higher retention of contaminants, it can also hinder desorption, reducing the reusability of the sorbent material[5]. Hence, finding an optimal balance between adsorption efficiency and sorbent regeneration is crucial for sustainable pesticide removal methodologies.
In recent years, carbon nanotubes (CNTs) have garnered significant attention as promising materials for pesticide adsorption and removal. CNTs exhibit exceptional adsorption properties due to their highly porous, hollow structures and extensive chemically active surface area [7]. These nanomaterials also possess the ability to generate induced dipoles, which facilitate the adsorption of organic molecules via Van der Waals interactions. Their hydrophobic nature further enhances their effectiveness in trapping and removing pesticide residues from aqueous and soil environments. Multi-walled CNTs, which consist of multiple layers of graphene sheets, are particularly advantageous as they provide additional molecular trapping mechanisms, making them highly versatile sorbents for environmental cleanup applications [7].
Beyond their application in pesticide adsorption, CNTs have also demonstrated remarkable efficacy in electrochemical sensor technologies for the detection and quantification of pesticide residues in food and environmental samples. Their unique electrical and structural properties enable the development of highly sensitive and selective detection systems, outperforming conventional analytical methods in terms of accuracy and efficiency [8]. Advanced CNT-based techniques, such as the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method, have further revolutionized pesticide extraction and detection processes, providing a robust and reliable alternative to traditional approaches [8].
To enhance the efficiency and practicality of CNT-based remediation techniques, researchers have developed magnetic CNT composites, which integrate CNTs with magnetic nanoparticles. These hybrid materials exhibit superior adsorption capabilities while allowing for easy separation and recovery using external magnetic fields. Such advancements have significantly improved the feasibility of large-scale pesticide removal, making CNT-based technologies highly attractive for environmental applications [8]. Given the increasing significance of CNTs in pesticide remediation and detection, a comprehensive review of their applications, advantages, and underlying mechanisms is imperative [9,10].
This review article aims to provide an in-depth analysis of the latest advancements in CNT-based pesticide removal strategies, highlighting their effectiveness, sustainability, and potential future research directions. By exploring the synergies between nanotechnology and environmental science, this review seeks to contribute to the development of innovative, eco-friendly solutions for mitigating pesticide contamination and ensuring environmental sustainability.
Fundamentals of Carbon Nanotubes (CNTs)
Carbon nanotubes (CNTs) have been at the forefront of nanotechnology research since their discovery in 1991, revolutionizing materials science with their remarkable structural and functional properties [11]. Composed of graphene sheets rolled into cylindrical structures, CNTs exist in two main forms: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs are characterized by a single graphene layer, typically measuring between 0.7 and 1.2 nm in diameter, while MWCNTs consist of multiple concentric graphene layers, ranging from 4 to 30 nm in diameter [7,8].
Despite their structural differences, both types exhibit similar thermal and electrical conductivities, making them suitable for a wide array of technological applications. SWCNTs have a purity of over 70% and are soluble in organic solvents but insoluble in aqueous solutions, while MWCNTs have a purity of over 95% and display aqueous solubility. The length of MWCNTs generally ranges between 30 and 50 nm in diameter, whereas SWCNTs fall between 0.7 and 1.2 nm. SWCNTs are poorly soluble in water due to their hydrophobic nature and tendency to aggregate, which limits their effectiveness in water treatment applications. To address this challenge, researchers employ covalent functionalization (e.g., introducing –COOH or –OH groups) to increase their hydrophilicity, although this can modify their intrinsic properties. Alternatively, non-covalent strategies involving surfactants, polymers, or aromatic compounds are used to disperse SWCNTs in water without altering their structure. These modifications significantly improve SWCNT dispersion, enabling better interaction with pollutants and enhancing their performance in water purification systems [12,13].
Synthesis Techniques of Carbon Nanotubes
The production of CNTs relies on various synthesis techniques, primarily categorized into plasma-based and thermal-based methods. Among the most widely employed techniques are arc discharge, laser ablation, and chemical vapor deposition (CVD), each offering distinct advantages in terms of yield, purity, and scalability [7,8].
Arc Discharge Method: This method involves applying a high voltage (approximately 20 V) between two graphite electrodes in an inert gas atmosphere, such as helium or argon, at a pressure of around 600 mbar. The electric arc generates temperatures exceeding 1700°C, leading to the vaporization of carbon atoms and their subsequent deposition onto the cathode, forming CNTs [11, 12]. The method is effective for producing both SWCNTs and MWCNTs, especially when combined with metal catalysts like iron, nickel, or cobalt to promote nanotube growth.
Laser Ablation Method: In this technique, a graphite target mixed with metallic catalysts is vaporized using a high-energy laser pulse in an inert gas environment at temperatures between 2700–3200°C. The laser ablation process allows precise control over CNT morphology, with synthesis parameters such as temperature, gas pressure, and catalyst composition significantly influencing the final nanotube characteristics [7,8]. However, the high energy requirements and low production yield limit its industrial scalability.
Chemical Vapor Deposition (CVD): The most widely used technique for large-scale CNT production, CVD involves the decomposition of hydrocarbon gases, such as methane, ethylene, benzene, carbon monoxide, or acetylene, over a metal catalyst at temperatures ranging from 600–1200°C. The lower temperature requirements and cost-effectiveness make CVD an ideal method for mass-producing CNTs [7,8]. This technique enables better control over nanotube alignment and structural properties, making it preferable for commercial applications.
Due to their unique electrical, thermal, and mechanical properties, CNTs have found extensive use across multiple industries, particularly in environmental applications. Their high surface area and tunable surface chemistry enhance their adsorption capabilities, making them highly effective in detecting and removing pesticides from water sources. The interaction mechanisms between CNTs and pesticides involve π-stacking, hydrogen bonding, electrostatic interactions, and coordination effects [5]. Functionalized CNTs exhibit superior adsorption efficiency due to the presence of active sites that facilitate chemical interactions.
MWCNTs have been particularly effective in pesticide removal due to their multi-layered structure, which provides enhanced stability and a higher density of active adsorption sites. Studies have demonstrated the effectiveness of SWCNTs in detecting organochlorine pesticides (OCPs), while MWCNTs have shown remarkable efficiency in adsorbing fenuron and heterocyclic pesticides. Moreover, the introduction of amine groups on CNT surfaces has improved the analysis of phenoxycarboxylic acid herbicides, while metal-modified CNTs have been utilized for extracting pesticides like Devrinol and triadimefon [5]. Additionally, metal-organic framework (MOF)-functionalized CNTs have been employed to remove pollutants such as chlortone and alachlor, highlighting their versatility in environmental remediation.
The continuous advancements in CNT synthesis and functionalization are driving their widespread adoption in environmental sustainability efforts. Their superior adsorption properties, cost-effectiveness, and stability make them a promising solution for pesticide detection and removal. Future research should focus on enhancing functionalization techniques to improve CNT selectivity and efficiency in addressing contamination challenges. As nanotechnology progresses, the role of CNTs in water purification and pollution control is expected to expand, reinforcing their significance in global environmental protection initiatives.
Among the available synthesis techniques, Chemical Vapor Deposition (CVD) stands out as the most suitable for large-scale production. Compared to arc discharge and laser ablation, CVD offers higher yield, better structural control, lower energy cost, and is already widely adopted in environmental applications like pesticide removal. Future research should focus on enhancing functionalization techniques to improve CNT selectivity and efficiency in addressing contamination challenges. As nanotechnology progresses, the role of CNTs in water purification and pollution control is expected to expand, reinforcing their significance in global environmental protection initiatives [14].
Structure and Properties of Carbon Nanotubes (CNTs)
Carbon nanotubes (CNTs) are defined as allotropes of carbon with a tubular structure, formed by rolling layers of graphene into cylindrical shapes. These nanostructures exhibit unique properties that make them highly suitable for various applications, including environmental remediation and pesticide removal. Based on their structural composition, CNTs are classified into two main types: single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) [9,10].
Structural Classification of CNTs
SWCNTs consist of a single graphene sheet rolled into a seamless cylinder with a diameter typically ranging from 0.4 to 2 nm. The structure of SWCNTs is defined by their chiral indices (n, m), which influence their electronic and mechanical properties. Depending on these indices, SWCNTs can exhibit three distinct configurations: armchair (n, n), zigzag (n, 0), and chiral (n, m). Each configuration determines the electrical conductivity, with armchair CNTs acting as metallic conductors, whereas zigzag CNTs exhibit semiconductor-like behavior (Figure 1) [15].
MWCNTs, on the other hand, consist of multiple concentric graphene cylinders nested within each other, resembling a tree-ring structure. These layers are held together by weak van der Waals forces, providing enhanced mechanical strength and thermal stability. MWCNTs can also include double- and triple-walled nanotubes, which further reinforce their robustness [15].
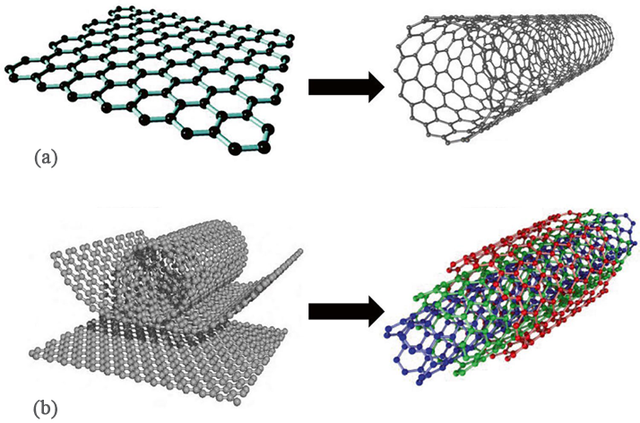
A graphene sheet can be used to synthesize different types of nanotubes by rolling the tubes down in various configurations. CNTs can take on unique structures, such as hollow graphite cylinders with hexagonally organized carbon rings, and have high tensile strength (~200 GPa), which is similar to that of graphene, but are more stable than graphene, even at extremely high temperatures, and optimize vibrational entropy. Due to the presence of multiple layers of carbon atoms, MWCNTs have higher mechanical strength compared with SWCNTs [16].
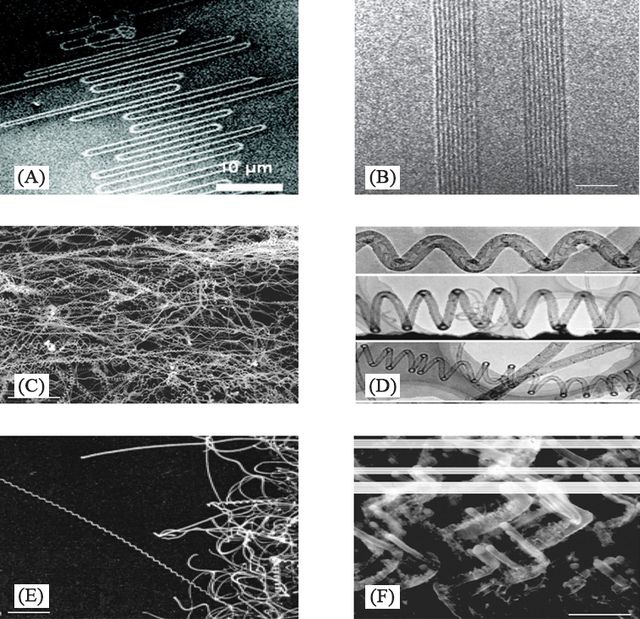
Scanning electron microscopy (SEM) describes the unusual zigzag shape, which is mainly due to SWCNT-substrate lattice interaction and gas flow. MWNTs comprise an array of such nanotubes that are concentrically nested like rings of a tree trunk, one of the main types of CNTs that can have high structural perfection. Coiled CNTs are produced by catalytic chemical vapor deposition (CVD) on an iron-coated indium tin oxide substrate. CNTs are found in helical forms more than 95% of the time. Each coil grows with its diameter and pitch. Transmission electron microscopy (TEM) is used to analyze the structure of the coiled CNTs. Similar to straight CNTs, coiled CNTs are built in a series of short tubes along the tube axis. The majority of the short tubes are bell-shaped, with one end capped and the other open, and are referred to as “nanobells.” SEM images show that a coiled CNT in a length of 20 mm long with a regular pitch extends out from the template. The zigzag morphologies consist of very sharp and alternating ∼90° bends. They were grown using a plasma-enhanced CVD process, where the bending of the CNTs during growth was caused by changing the direction of the electric field lines in the growth region of the sample (Figure 2) [16].
Electronic and Thermal Properties
The electronic properties of CNTs are largely governed by their chirality and diameter. Armchair CNTs with a chiral index of (n, n) exhibit metallic behavior, whereas zigzag and certain chiral configurations act as semiconductors. This tunability of electrical properties makes CNTs highly valuable for nanoelectronic applications, including sensors and transistors [17].
CNTs also possess remarkable thermal conductivity due to the presence of sp2-hybridized covalent bonds. SWCNTs exhibit thermal conductivity values ranging from 1800 to 6000 W/m·K, surpassing that of diamond, which was previously considered the highest thermally conductive material. MWCNTs also demonstrate excellent thermal properties, with values around 3000 W/m·K. These attributes make CNTs suitable for applications requiring efficient heat dissipation, such as thermal management in nanocomposites and electronic devices [17].
Mechanical Strength and Structural Integrity
The mechanical properties of CNTs are exceptional, attributed to the strength of their sp2-hybridized carbon-carbon bonds. They can withstand significant mechanical stress without permanent deformation, returning to their original structure once the force is removed. The Young’s modulus of SWCNTs typically reaches ~1.25 TPa, significantly higher than that of bulk graphite. Their tensile strength can reach up to 150 GPa, making them among the strongest materials known [9,10].
The mechanical strength of CNTs is influenced by factors such as chirality, length, and diameter. Studies suggest that zigzag CNTs exhibit higher tensile strength under uniaxial loading than armchair CNTs. While the diameter significantly impacts electrical conductivity, it has minimal influence on tensile strength, making CNTs reliable for structural reinforcement in nanocomposites. MWCNTs, due to their multilayered structure, provide better mechanical resilience and durability compared to SWCNTs.
The extremely high Young’s modulus of SWCNTs (approximately 1–5 TPa) further highlights their superior mechanical properties, placing them among the stiffest materials known. This exceptional stiffness plays a crucial role in two key application areas:
Filtration Systems:
The high stiffness enables the fabrication of ultra-thin yet mechanically stable filtration membranes. These membranes can withstand high pressure differentials during filtration without bending, cracking, or deforming. For instance, in nanofiltration or microfiltration systems, membranes incorporating SWCNTs maintain their structural integrity under operational stress, ensuring consistent flow and long-term performance. In contrast, membranes made from other nanomaterials may collapse or lose shape under pressure.
Sensors (e.g., NEMS):
In nanoelectromechanical systems (NEMS), SWCNTs serve as critical components such as resonators or cantilevers. Their high Young’s modulus allows them to vibrate at very high frequencies, enabling ultra-sensitive detection of mass or force changes, down to single molecules. This makes them ideal for environmental sensing applications, such as detecting trace amounts of pesticides or pollutants in water. The mechanical stability ensures reliable sensor performance even under varying environmental or mechanical conditions.
Additionally, when SWCNTs are embedded into composite materials (e.g., polymer-SWCNT nanocomposites), they significantly enhance the mechanical strength and stiffness of the host matrix. This results in lightweight yet durable materials well-suited for use in portable or flexible filtration devices and high-performance sensors [15,18].
Surface Properties and Functionalization Potential
The surface properties of CNTs play a crucial role in their effectiveness for various applications, particularly in environmental remediation. Their high aspect ratio and large surface area contribute to superior adsorption capabilities. CNTs exhibit strong π-π interactions and hydrophobicity, making them ideal for adsorbing organic pollutants such as pesticides [16].
Functionalization of CNTs through oxidation and chemical modifications introduces oxygen-containing groups onto their surfaces, improving their dispersibility in aqueous solutions and enhancing their ability to bind with contaminants. This functionalization is essential for optimizing CNTs in filtration systems and adsorption-based applications, ensuring higher selectivity and efficiency in pollutant removal [9,10].
The distinctive structural and physicochemical properties of CNTs make them versatile materials with immense potential in numerous applications, including pesticide removal. Their remarkable electronic, thermal, and mechanical characteristics, coupled with tunable surface properties, provide a strong foundation for their role as effective nanomaterials in environmental remediation. By leveraging their high adsorption capacity and functionalization potential, CNTs can be strategically engineered to enhance pollutant removal efficiency, paving the way for sustainable and advanced filtration technologies [9,10].
Unique Properties Relevant to Adsorption (Surface Area, Hydrophobicity, etc.)
Carbon nanotubes (CNTs) have gained widespread recognition for their exceptional adsorption properties, making them highly effective materials for wastewater treatment and environmental remediation. Their unique structure, consisting of graphene sheets rolled into cylindrical nanotubes, provides a high surface area, tunable porosity, and the ability to interact with various contaminants via π-π stacking, van der Waals forces, hydrophobic interactions, hydrogen bonding, and electrostatic forces [12,13]. These interactions are dependent on the properties of the target compounds and the functionalization of CNTs.
Adsorption Mechanisms and Efficiency
Among carbon-based nanomaterials, CNTs exhibit superior adsorption capacities due to their high surface area, controlled pore size, and active adsorption sites. Single-walled CNTs (SWCNTs) have been observed to possess particularly high adsorption efficiency for organic contaminants due to their large micropore volume and extensive surface area[19]. The adsorption of pollutants onto CNTs is influenced by several factors, including the specific surface area, functional groups, purity, and the availability of adsorption sites. These factors determine the extent of interaction between CNTs and contaminants [20].
In aqueous environments, CNTs tend to aggregate, forming interstitial grooves and spaces that enhance their adsorption capabilities[19]. Studies have demonstrated that modifying CNTs with functional groups such as oxygen, nitrogen, and sulfur significantly enhances their adsorption efficiency by introducing hydrophilic or electron-donating properties [13,14]. For instance, nitrogen-doped CNTs have shown improved adsorption performance for organic pollutants like tylosin, tetracycline, and bisphenol-A due to increased electronic polarization (Figure 3)[19].
Functionalized and Composite CNTs for Enhanced Adsorption
CNTs have been successfully incorporated into nanocomposites, such as CNTs-graphene, CNTs-magnetic graphene, and CNTs-bentonite, to improve adsorption performance [19]. These composites provide additional functionalities that enhance adsorption efficiency, pollutant selectivity, and reusability. Additionally, reactive metals and oxides embedded in CNTs generate highly reactive oxygen species that can degrade harmful organic contaminants into harmless byproducts like carbon dioxide and water [19].
Oxidized CNTs further enhance adsorption through functionalization with ester groups or doping with metal-organic frameworks (MOFs), leading to superior adsorption capacities. A study demonstrated that oxidized CNTs functionalized with pentaerythritol (PER) exhibited an adsorption capacity of 257.73 mg/g for organic dyes. Similarly, multi-walled CNTs (MWCNTs) embedded in MOFs have shown excellent adsorption performance for tetracycline antibiotics, highlighting the versatility of CNT-based adsorbents [19,22,23].
Adsorption of Atrazine and Other Contaminants
Recent studies have explored the application of CNTs for pesticide removal, particularly atrazine, an herbicide known for its environmental persistence. The adsorption capacity of CNTs towards atrazine is influenced by surface area, pore structure, and functional groups. Due to their sp²-hybridized carbon atoms, CNTs effectively adsorb aromatic compounds like atrazine via π-π electron donor-acceptor (EDA) interactions. Additionally, hydrophobic effects, weak dipolar forces, and dispersion interactions contribute to the adsorption process [21].
Experimental studies have reported that reduced MWCNTs (r-MWNTs) exhibit the highest atrazine adsorption capacity, with values as high as 110.8 mg/g at 18°C [21].
Despite the high adsorption efficiency of CNTs, challenges remain in their practical application. The presence of oxygen-containing functional groups, such as carboxyl and hydroxyl groups, significantly influences adsorption behavior by increasing hydrophilicity, which can either enhance or inhibit contaminant uptake. For example, one study evaluated the use of CNTs for removing pesticides such as atrazine, simazine, and diuron from water. The findings showed that CNTs achieved high removal efficiencies—exceeding 90%—highlighting their strong adsorption capacity and potential use in water treatment processes. This supports the feasibility of CNTs as high-performance adsorbents under semi-realistic conditions.
To improve practicality and scalability, researchers have explored combining CNTs with magnetic nanoparticles to form nanocomposites. These composites enable easy separation of the adsorbent from water using external magnets, making them more suitable for field application. Studies have shown that such magnetic CNT-based adsorbents can be reused multiple times with minimal loss in efficiency, addressing some of the regeneration and handling challenges that often limit nanoparticle use in the field.
These pilot-scale investigations show that CNTs and CNT-based composites offer promising capabilities for pesticide removal in practical water treatment settings. However, further work is still required to optimize their cost-effectiveness, environmental safety, and long-term performance in large-scale applications [24,25].
Issues such as aggregation, high production costs, and potential recontamination risks need to be addressed. The regeneration of CNTs, an essential factor in their cost-effectiveness, can be achieved by adjusting the solution’s pH using acids like nitric acid (HNO₃)[19]. Furthermore, CNT-based membranes and composites have been explored as solutions to overcome these limitations, providing enhanced separation and adsorption efficiency while mitigating membrane fouling issues [19].
The functionalization of CNTs continues to be a promising approach to improve their selectivity and adsorption capacity for specific contaminants. Future research should focus on optimizing the modification of CNTs to enhance their performance in multi-component systems, addressing the complexities of natural wastewater interactions. The integration of CNTs with magnetic materials, such as Fe₃O₄ and iron metal-organic frameworks, has also proven beneficial in facilitating the easy separation and reuse of CNT-based adsorbents [19].
Carbon nanotubes remain at the forefront of nanotechnology research for adsorption applications, owing to their high surface area, tunable porosity, and unique interaction mechanisms with pollutants. While challenges such as aggregation and high production costs persist, advancements in functionalization and composite formation continue to improve their performance. As research progresses, CNT-based materials are expected to play a crucial role in environmental remediation, particularly in wastewater treatment and pollutant removal.
Functionalization and Modification of CNTs for Enhanced Performance
Carbon nanotubes (CNTs) have gained significant attention in recent years due to their unique physicochemical properties, including high mechanical strength, superior electrical conductivity, excellent thermal stability, large surface area, and controllable surface structure [26]. However, their inherent chemical inertness and tendency to aggregate in solution limit their applicability in adsorption-based processes, including the removal of pesticides. Functionalization and modification of CNTs are essential strategies to enhance their performance by improving their dispersibility, introducing active adsorption sites, and increasing their chemical reactivity.
Functionalization of CNTs can be achieved through mechanical, irradiation, and physicochemical methods, with physicochemical methods further categorized into covalent and non-covalent functionalization [27]. Covalent functionalization involves altering the sp2 hybridization of CNTs to sp3, introducing functional groups that improve adsorption properties. This covalent bonding disrupts the sp2 structure to sp3, promoting lattices (defects) in the CNT structure, modifying some of their properties (electrical, optical, thermal). The surface chemical modification of CNTs allows their reaction with other chemical groups, enhancing their reactivity and expanding the CNT applications, besides their customization for a specific purpose [28].
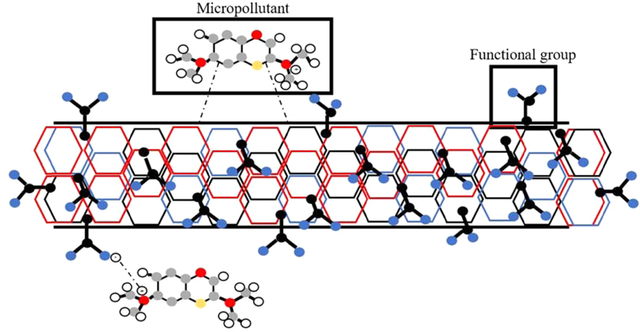
Non-covalent functionalization, on the other hand, retains the intrinsic properties of CNTs while enabling interaction with various functional groups. These modifications reduce aggregation and enhance CNTs’ interaction with target pollutants. The non-covalent bonding preserves the sp2 hybridization and π-conjugated structure of the CNTs, and also their optical and electronic properties. The chemical interactions here are weaker (CH-π, electrostatic, hydrogen bonding, ionic bonds, π-π, van der Waals) and possibly reversible, to maintain organic and/or inorganic molecules adsorbed on the CNT surface (Figure 4)[28].
Covalent and non-covalent functionalization of carbon nanotubes (CNTs) significantly enhances their adsorption performance, and the selection of the method depends on the chemical nature of the target pesticide.
Covalent functionalization introduces polar functional groups (e.g., –COOH, –OH, –NH₂) onto the CNT surface. These groups improve hydrophilicity and enable hydrogen bonding and electrostatic interactions, making this approach particularly effective for polar pesticides, such as organophosphates. Additionally, imidazole-functionalized CNTs show enhanced binding to organophosphates through strong coordination interactions.
Non-covalent functionalization preserves the intrinsic structure and electrical conductivity of CNTs. It relies on π–π stacking and hydrophobic interactions, which are ideal for adsorbing non-polar, aromatic pesticides like organochlorines. Techniques such as polymer wrapping (e.g., using poly(3-hexylthiophene)) further enhance dispersion and binding surface availability.
Covalent modification is more effective for polar pesticides (e.g., organophosphates) due to specific chemical interactions, while non-covalent functionalization is suited for non-polar, aromatic pesticides (e.g., organochlorines) through physical affinity mechanisms.
The incorporation of oxygen-, nitrogen-, and sulfur-containing functional groups significantly affects CNT adsorption efficiency. Oxygen-containing groups such as hydroxyl, carboxyl, and carbonyl increase hydrophilicity, improving CNT dispersion in aqueous solutions and enhancing adsorption capacity via electrostatic interactions and hydrogen bonding [26]. Nitrogen functionalization introduces basic sites, improving interactions with acidic pollutants and strengthening metal adsorption capabilities. Furthermore, sulfur-containing functional groups enhance the selectivity of CNTs for certain metal pollutants, though they may affect the surface area and pore structure depending on the modification process.
Acid treatment is a commonly used modification technique to introduce negatively charged functional groups on CNT surfaces, which improves their affinity for positively charged metal ions and organic pollutants [27]. The oxidation process not only increases surface oxygen content but also assists in opening closed CNT ends, creating more active adsorption sites. Additionally, composite formation with metal oxides such as alumina, ZnO, and Fe2O3 has been extensively explored to enhance CNT adsorption efficiency. For instance, the combination of multi-walled carbon nanotubes (MWCNTs) with iron oxide significantly improves the removal capacity of metallic pollutants and facilitates easy separation from aqueous solutions [26].
Functionalization plays a crucial role in optimizing CNT adsorption mechanisms. Depending on the nature of the pollutant, interactions may involve physical adsorption, electrostatic interactions, ion exchange, surface complexation, or π–π stacking [27]. The introduction of basic nitrogen groups, such as pyridine N and pyrrole N, has been found to enhance catalytic efficiency, making functionalized CNTs effective in CO2 capture and transformation. Such basic sites facilitate CO2 adsorption and lower molecular conversion barriers, leading to improved performance in catalytic reactions [26].
The effectiveness of CNT-based adsorbents in pesticide removal largely depends on the density and type of functional groups, pH of the solution, and ionic strength. For instance, increasing the number of oxygen-containing functional groups enhances CNT interaction with polar pesticides through hydrogen bonding and electrostatic attraction [27]. Moreover, surface modifications aimed at increasing microporosity improve CNT adsorption performance by expanding available binding sites.
The functionalization and modification of CNTs are crucial for improving their adsorption efficiency and broadening their application in environmental remediation, including pesticide removal. Future research should focus on optimizing modification techniques to maximize adsorption performance while maintaining the structural integrity of CNTs. These advancements will enable CNTs to serve as highly effective and selective adsorbents for pesticide removal, contributing to the development of sustainable water treatment technologies [28].
Applications of CNTs in Pesticide Removal
Carbon nanotubes (CNTs) have gained significant attention in agricultural applications due to their exceptional physicochemical properties, such as large surface area, high reactivity, and nanoscale dimensions. These properties make them suitable for various applications, including plant growth enhancement, pesticide removal, and biosensor-based diagnostics. However, their potential toxicity and long-term environmental impact require further investigation [16].
CNTs in Plant Growth
CNTs have been explored for their role in promoting seed germination and early plant development. Studies suggest that CNTs can penetrate the seed coat, facilitating water uptake and increasing moisture retention, thereby accelerating seed germination and early growth. For instance, oxidized MWCNTs have been found to enhance water absorption in mustard seeds, leading to a higher germination rate and increased moisture content compared to untreated seeds [16]. This effect is believed to be associated with the interaction of CNTs with aquaporins, which regulate water transport in plant cells.
Research on various crops, including tomato, rice, cucumber, and soybean, has demonstrated that CNTs at concentrations between 50–100 mg/L can effectively penetrate seed structures, promoting faster growth rates. However, factors such as CNT size, shape, surface chemistry, and functionalization influence their impact on plant development. Some studies have also raised concerns about potential toxicity, particularly when CNTs interact with heavy metals like cadmium (Cd), which can hinder root growth and stress plant defense mechanisms [17].
CNTs in Pesticide Analysis
Due to their high adsorption capacity, CNTs have been extensively used in analytical techniques such as solid-phase extraction (SPE) and solid-phase micro-extraction (SPME). These methods enable the detection and removal of pesticides, heavy metals, and organic contaminants. MWCNT-based sorbents have shown remarkable efficiency in pre-concentrating organophosphate pesticides and chlorophenols, demonstrating high recovery rates under optimized conditions [16].
Functionalized CNTs, such as carboxylated SWCNTs, exhibit superior adsorption performance due to their increased affinity for pesticide molecules. Studies comparing CNT-coated fibers with conventional polymer coatings, such as polydimethylsiloxane (PDMS), have reported significantly higher extraction efficiency for CNT-based sorbents. Additionally, hybrid nanomaterials, including CNTs/SiO₂ composites, have further enhanced pesticide detection sensitivity, improving analytical precision and detection limits [17].
CNTs in Pesticide Removal
The unique structural features of CNTs contribute to their effectiveness in pesticide removal. The high surface area of CNTs enables strong interactions with pesticide molecules via π-π stacking, hydrophobic interactions, and electrostatic forces. Reduced MWCNTs (r-MWCNTs) have demonstrated high adsorption capacities for pesticides such as atrazine, with values reaching up to 110.8 mg/g at 18°C. The presence of oxygen-containing functional groups has been shown to enhance adsorption, although excessive functionalization can reduce efficiency due to increased hydrophilicity [16, 28,17].
Potential Risks and Future Directions
Despite their promising applications, the potential risks of CNTs in agriculture must be carefully assessed. The ability of CNTs to accumulate in plant tissues and alter physiological processes raises concerns regarding food safety and environmental sustainability. Functionalization strategies and controlled CNT concentrations should be optimized to maximize benefits while minimizing adverse effects [17].
Innovative CNT-based materials, such as Fe₃O₄-modified magnetic CNTs, have been developed to facilitate the easy separation and reuse of CNTs in water treatment applications. These magnetic composites enable CNT recovery through external magnetic fields, improving their practicality for large-scale pesticide removal. However, some studies indicate that while magnetic CNTs enhance separation efficiency, they may exhibit lower adsorption capacities due to modifications on the CNT surface.
Magnetic carbon nanotube (CNT) composites offer notable advantages such as ease of separation and reusability. They can be rapidly separated from aqueous solutions using an external magnetic field, eliminating the need for filtration or centrifugation and facilitating easier recovery and potential reuse of the adsorbent. Additionally, their magnetic properties support efficient regeneration and reuse, helping reduce long-term operational costs. However, this approach also has trade-offs. The incorporation of magnetic particles may slightly reduce the available surface area for adsorption compared to pristine CNTs, resulting in a modest reduction in adsorption capacity. Moreover, the synthesis of magnetic CNT composites involves additional steps and materials, potentially increasing the initial production costs.
On the other hand, non-magnetic CNTs provide their own advantages. They exhibit high adsorption efficiency due to their pristine high surface area and the ability to be functionalized for enhanced pesticide removal. The synthesis process for non-magnetic CNTs is generally simpler and more cost-effective than that of magnetic composites. Nonetheless, they come with limitations. After adsorption, separating non-magnetic CNTs from water typically requires additional processes like filtration or centrifugation, which can be time-consuming and less efficient. These separation challenges may also hinder the effective regeneration and reuse of the adsorbent [29].
Future research should focus on improving the selectivity of CNTs for pesticide adsorption, enhancing regeneration techniques, and integrating CNTs with sustainable materials. The development of CNT-based biosensors for real-time pesticide detection could provide a valuable tool for monitoring agricultural contaminants. Additionally, further studies on the long-term ecological impact of CNTs are necessary to ensure their safe application in agriculture [16].
Carbon nanotubes hold immense potential for pesticide removal, enhancing plant growth, and various agricultural applications. Their high adsorption capacity, tunable surface properties, and nanoscale dimensions make them valuable tools for improving agricultural productivity and environmental sustainability. However, challenges related to toxicity, cost-effectiveness, and large-scale implementation must be addressed through continued research and innovation. The integration of CNTs into advanced pesticide remediation systems and biosensing platforms could revolutionize the field of agricultural nanotechnology in the future.
Removal of Organochlorine Pesticides
Organochlorine pesticides (OCPs) have been extensively used to combat pests across various agricultural sectors due to their effectiveness in disrupting the neural functions of target organisms. Despite their ban in many developed countries, the persistence of these chemicals in the environment continues to pose significant risks to both ecological and human health. The structural stability of OCPs, attributed to the strong carbon-chlorine bonds in their aromatic rings, enables them to remain in the environment for extended periods, resisting natural degradation processes such as hydrolysis. This resilience is further bolstered by factors such as pH, temperature, ionic strength, and the presence of metal ion catalysts [30].
The ubiquitous presence of OCPs in water, soil, and air—detected even in remote locations like deserts and snowy landscapes—highlights their volatile nature and capacity for long-distance dispersion. Examples of common OCPs include DDT, aldrin, dieldrin, and hexachlorobenzene, with newer variants such as imidacloprid recently reported [30]. Their detection and classification are typically based on the pest or organism they target, ranging from herbicides to nematicides, and by their chemical composition, including compounds like dibenzo-p-dioxins and polychlorinated biphenyls [30].
Given their toxic nature and environmental persistence, the removal of OCPs is crucial. Traditional adsorption techniques have been employed extensively for this purpose. Activated carbon, for instance, has been widely used to cleanse water contaminated with pesticides, proving effective in various settings, from pesticide production facilities to drinking water treatment systems. Alternative adsorbents such as chitosan and zeolite have also demonstrated capability in removing OCPs from aqueous solutions, with recent studies exploring the efficacy of graphene oxide in adsorbing these persistent pollutants [31].
In response to the challenges posed by organochlorine pesticides, there is a growing interest in leveraging the unique properties of carbon nanotubes (CNTs) for environmental remediation. The large surface area, chemical stability, and modifiable surface characteristics of CNTs make them potentially superior adsorbents for capturing and degrading OCPs. Future research should focus on enhancing the functionalization of CNTs to target specific OCPs, optimizing their interactions with these complex molecules, and scaling up these technologies for practical, real-world applications.
Challenges and Limitations
While carbon nanotubes (CNTs) are celebrated for their remarkable physicochemical properties, such as their diminutive size, expansive surface area, and the capacity to penetrate cellular barriers, they also bring significant challenges and limitations that must be addressed. These challenges are particularly pronounced in agricultural applications where CNTs have shown potential to enhance plant growth, improve nutrient delivery efficiency, and increase crop resilience to environmental stresses [17].
Despite these benefits, several significant barriers hinder the broader adoption of CNT-based technologies in agriculture. One of the primary concerns is the phytotoxicity associated with CNTs. Recent studies have explored strategies to mitigate the phytotoxic effects of carbon nanotubes (CNTs) while maintaining their effectiveness in pesticide removal. For instance, one study investigated the use of multi-walled carbon nanotubes (MWCNTs) on rice plants under cadmium (Cd) stress and found that applying MWCNTs at optimized concentrations, such as 500 mg kg⁻¹, reduced Cd accumulation in plant tissues and soil. Additionally, MWCNTs lowered oxidative stress markers like malondialdehyde (MDA) and antioxidant enzyme activity in leaves, indicating reduced toxicity. These findings suggest that adjusting CNT concentrations can minimize phytotoxicity without compromising pollutant adsorption.
In addition to concentration control, functionalizing CNTs with biocompatible groups has been shown to reduce their toxicity. For example, carboxylated MWCNTs improve dispersion and reduce aggregation, thereby enhancing pesticide adsorption and lowering their harmful effects on plants. Coating CNTs with biocompatible polymers such as polyethylene glycol (PEG) also helps minimize direct interaction with plant cells and reduces oxidative stress. Furthermore, recent studies propose surface coating with biocompatible polymers (e.g., PEGylation) or functionalization with antioxidant molecules to reduce reactive oxygen species (ROS) production and mitigate CNT phytotoxicity.
Another promising strategy involves using CNTs as carriers for controlled pesticide delivery. Encapsulating pesticides within CNTs enables targeted application to plant tissues, reducing off-target exposure and phytotoxicity. This method not only enhances pesticide efficiency but also leverages the high adsorption capacity of CNTs.
Overall, phytotoxicity can be mitigated by optimizing CNT concentration, applying surface functionalization with biocompatible groups, and employing CNTs as controlled delivery systems. These strategies offer promising solutions for safer and more efficient use of CNTs in agricultural and environmental applications[32,10].
Their small size and large surface area facilitate not only beneficial interactions with plant cellular structures but also potentially harmful ones. Studies have indicated that CNTs can induce oxidative stress within plant cells, alter gene expression, and ultimately affect plant vitality by reducing cell viability, delaying flowering, curtailing root growth, and causing wilting and discoloration of leaves. In some cases, these effects culminate in yield reduction or even plant death through mechanisms such as apoptosis. Moreover, the environmental impact of CNTs extends beyond the plant cells to the soil ecosystem. CNTs can accumulate in the soil, potentially disrupting microbial diversity and harming beneficial microbial populations. This accumulation can alter the soil microbiome, which is essential for nutrient cycling and plant health. Additionally, multi-walled carbon nanotubes (MWCNTs) have been found to act as carriers for other contaminants, raising concerns about the potential for bioaccumulation of hazardous substances in crops, which may vary depending on the contaminants’ concentration on the MWCNTs.
Another significant challenge is the economic viability of using CNTs in agriculture. Recent studies have examined the economic viability of carbon nanotube (CNT)-based adsorbents compared to traditional methods like activated carbon (AC) filtration in water treatment applications. Activated carbon is widely used due to its affordability and effectiveness, with prices typically ranging from $500 to $3,000 per ton depending on quality and source. In contrast, CNTs—including single-walled and multi-walled types—are significantly more expensive, primarily due to the complexity of their synthesis and the cost of purification.
Despite the higher initial cost, CNTs offer notable advantages in terms of adsorption capacity and reusability. Studies have demonstrated that CNTs can be reused through multiple treatment cycles without a significant decline in performance, which may help offset their initial expense over time. Although CNT-based adsorbents are currently more costly than AC, their superior performance characteristics can justify the investment in applications where high efficiency and long-term use are critical. Nevertheless, for large-scale or cost-sensitive operations, activated carbon remains the more economically viable choice.
The production costs of single-walled and multi-walled carbon nanotubes are currently higher than those of conventional agricultural products, such as fertilizers and antimicrobial agents. This cost disparity must be overcome to make CNTs a competitive alternative in agricultural practices.
The scalability of CNT synthesis and their reusability also pose considerable challenges. While the unique properties of CNTs are highly beneficial, the ability to produce them on a large scale and reuse them effectively in agricultural settings without loss of functionality or further environmental impact needs more research.
Current research is still exploring the myriad interactions between CNTs and plant systems, indicating that this field is in its early stages. Key areas requiring further investigation include the mechanisms of water uptake enhancement by CNTs during seed germination, the behavior and translocation patterns of CNTs within plant systems, and the specific interactions at the CNT-plant cell interface under stress conditions. Additionally, the potential genetic modifications induced by CNTs and their implications for plant health and productivity warrant thorough examination [11,12].
Given these concerns, it is crucial that any application of CNTs in agriculture includes a comprehensive toxicity assessment. This assessment should extend to evaluating the safety of consuming plants exposed to CNTs, as these materials can be transferred to humans through the food chain. Developing reliable and effective methods to assess and mitigate the ecotoxicological impacts of CNTs is essential for ensuring that their use in agriculture does not compromise environmental health or food safety [17].
Conclusion
The remarkable attributes of CNTs, including their extensive surface area, reactivity, and modifiable structure, position them as key assets in the efficient extraction and detection of pesticide residues from environmental contexts.
CNTs’ capacity for enhancement through functionalization and their compatibility with composite materials enhances their utility, making them versatile tools for tailored environmental remediation strategies. Innovations such as magnetic CNT composites demonstrate the potential for practical, reusable solutions in large-scale applications, aligning with sustainable environmental practices.
However, the deployment of CNTs is accompanied by significant challenges. The concerns regarding their potential ecological and health impacts, along with issues related to cost and production scalability, must be addressed through comprehensive research and strategic development efforts.
Looking forward, research should concentrate on improving the specificity and adsorption efficiency of CNTs toward particular pesticide targets. Enhancing the economic feasibility of these technologies is also critical for their adoption at a broader scale. Moreover, thorough assessments of the long-term environmental impacts of CNTs are crucial to ensure their sustainable application. As advancements continue, it is expected that CNT-based innovations will increasingly become integral to developing sophisticated solutions for managing pesticide pollution. This will not only aid in boosting agricultural yields but also in safeguarding environmental and public health, setting the stage for a future where nanotechnology plays a central role in sustainable practices.
To support this progress, functional groups such as carboxyl (-COOH) and hydroxyl (-OH) have been introduced onto CNT surfaces to increase their affinity for polar pesticides. These groups enhance interactions like hydrogen bonding and electrostatic attractions, making CNTs more effective in adsorbing specific pesticide molecules. Additionally, composite materials combining CNTs with metal-organic frameworks (MOFs) or magnetic nanoparticles have been explored. For instance, integrating CNTs with MOFs can create structures that offer both high surface area and specific binding sites, improving the removal of pesticides from contaminated water[9].
Statements and Declarations
Competing Interests
The author declares no conflicts of interest.
Ethics approval
Not applicable.
Data availability
Not applicable.
Funding
No funding was received to support this work.
Authors’ Information
Azam Serajian—Department of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, Iran.



 - Copy copy.png)


