Visual Abstract
Abstract
The dimeric silicotungstate, [(B-α-SiW9O34)2Cd4(H2O)2]12- (I), has been synthesized in a new and simple method by reaction of A-b-Na9HSiW9O34‧23H2O with cadmium nitrate under controlled temperature conditions. I was characterized by elemental analysis, FT-IR, 29Si, and 113Cd NMR spectroscopy, and this was verified by single-crystal X-ray structural analysis of the combined salts of Na+ and NH4+. The (NH4)10Na2[(B-α-SiW9O34)2Cd4(H2O)2]·22H2O salt crystallizes in the monoclinic system (space group P21/n) with a = 16.2706(18) Å, b = 13.8262(17) Å, c = 20.307(3) Å, b= 113.419(2)° and Z = 2. Two lacunary B-α-[SiW9O34]10- Keggin moieties are connected by a rhomboid Cd4O16 group to form the heteropolyanion, which has a sandwich-like shape. Cyclic voltammetry was used to examine the electrochemical and electrocatalytic properties of I in an aqueous solution. The redox processes of WVI and CdII centers are represented by the two sequential cathodic/anodic peaks observed in electrochemical experiments. The redox waves of I are pH-dependent. For this compound, the tungsten and cadmium-centered waves exhibit a classical potential shift at pH values between 1.6 and 4.6 as a function of acidity. I electrocatalysis tendencies toward the reduction of nitrite ions have been thoroughly investigated. Additionally, in this study, the cytotoxic and apoptogenic activity of several sandwiched-type polyoxometalates (I, ([(B-α-PW9O34)2Cd4(H2O)2]10- (II), [(B-α-PW9O34)2Mn4(H2O)2]10- (III), [(B-α-PW9O34)2Co4(H2O)2]10- (IV), [(B-α-PW9O34)2Zn4(H2O)2]10- (V), [(ZnW9O34)2WZn3(H2O)2]12- (VI), [(ZnW9O34)2WCd3(H2O)2]12- (VII), and [(B-α-PW9O34)2Zn2Cd2(H2O)2]10- (VIII)) were tested on four types of human cancer cells (MCF-7, B16F10, DU-145, and PC3). All tested compounds showed cytotoxic activity which is mediated via apoptosis.
Sandwich-type polyoxometalates, 113Cd NMR spectroscopy, 29Si NMR spectroscopy, Electrochemistry, Cytotoxicity, Apoptosis
Introduction
The possibility of using polyoxometalates (POMs) in catalysis, medicine, and material science has greatly sparked interest in the chemistry of these compounds [1–4]. Among these clusters, one of the most interesting groupings is the transition metal-substituted polyoxometalates (TMSPs). These compounds can be created by inserting a heteroatom (or, more frequently, substituting one or more addenda atoms) with lower valent transition metals. The Keggin and Wells-Dawson structures form the foundation for the most prevalent kinds of TMSPs [5, 6].
A subgroup of TMSPs has been investigated in recent years by research teams studying heteropolyanions. These compounds can be seen as having transition metals "sandwiched" between two trivacant forms of the parent Keggin or Wells-Dawson structures [7, 8]. As an illustration, a large number of tetra nuclear sandwich-type complexes [[M4(H2O)2(PW9O34)2]10- (abbreviated as P2W18M4) are known with different divalent metal ions (MΙΙ = Mn, Co, Cu, Zn, Cd) [9, 10].
Recently, the possibilities of preparing dimeric or sandwiched compounds of heteropolyanions containing [SiW9O34]10- unit were studied. Subsequently, the anions [Zr3(H2O)2(SiW9O34)2]11-, [Si2W18Ti6O77]14-, [Cr6(OH)9(SiW9O34)2]11- and [Sn3(SiW9O34)2]14- have been synthesized [11–14].
In 2000, Kortz et al. reported some tetra nuclear sandwiched-type complexes of silicotungstates based on the lacunary Keggin ion, [(SiW9O34)2M4(H2O)2]12- (MΙΙ = Mn, Cu, Zn) [15]. The synthesis of these compounds relies on the interaction between transition metal ions and [γ- SiW10O36]8-. In 2013, Jiuyu Guo et al. discovered that the dimerization of [SiW9O34]10- anions forms the open Wells–Dawson anion [Si2W18O66]16- [16]. Also, another sandwiched-type compounds based on [SiW9O34]10- anions (TBA7H2[Ag2(SiW9O31)2(CH3COO)3]·H2O·CCl4 and [TBA8H6Ag6(SiW9O33)2(DMF)]2+) reported by Kentaro Yonesato et al [17]. The use of POMs in reducing nitrite, iodate, hydrogen peroxide, and bromate has been the subject of numerous investigations during the past decades [18, 19]. Although most electrode surfaces require a significant overpotential for direct electro-reduction of nitrite ions, sandwich-type POMs have lately been able to reduce NO2- electro-catalytically with much less effort [20].
Cancer, one of the leading causes of death worldwide, encompasses a range of disorders in which cells grow uncontrollably, forming tumors or causing blood cancers, with the most common types being lung, colon, prostate, and breast cancer [21–23]. Even though cancer treatment has advanced over time, distant metastasis and tumor recurrence rates are still significant [24]. Conventional cancer treatment includes surgery, hormone therapy, radiation, and chemotherapy [25]. Chemotherapy is one of the most popular cancer therapies, with the main objective of eradicating and reducing the proliferation of cancer cells through the administration of one or more anticancer medications [26]. One of the primary mechanisms of action of chemotherapy may be the induction of apoptosis, a genetically set process that results in cells dying [27, 28]. The major drawbacks of chemotherapy drugs include severe side effects caused by a lack of selectivity, ineffectiveness against specific cancer types, and low absorption. Therefore, searching for alternative medications that selectively disable cancer cells without severely harming normal cells continues [21, 29].
Recently, along with the rapid development of the structures of the POMs, these compounds are ideally suited for biological applications owing to their promising anti-tumor [30–32], anti-viral [33, 34], anti‐bacterial [35–37], anti-fungal [38, 39], anti-diabetes [40, 41], anti-Alzheimer’s [41], skin anti-aging [42], and cancer diagnosis [30, 31] activities. Although the exact processes by which POMs exert their biomedical applications are not still clear, they probably catalyze Fenton-like reactions to increase ROS levels and possess anti-inflammatory and immunomodulatory properties [43–45]. POMs can also be used as biosensors [46, 47]. The first reports of anticancer, antiviral, and antibacterial activities of POMs were in 1965, 1971, and 1993, respectively [48]. Since then, many POMs, polyoxotungstates in particular, have been extensively studied for their activities in vitro [49].
In this work, we describe the synthesis and crystal structure of another member of sandwich-type polyoxometalates. This new cadmium-containing heteropolyanion was synthesized by a reaction of [A—SiW9O34]10- unit which surrounds four cadmium. Besides the characterization of I, this compound’s electrochemistry, electrocatalytic, and photocatalytic properties were studied. Additionally, AlamarBlue® cell proliferation assay was used to assess the cytotoxic activity of ([(B-α-SiW9O34)2Cd4(H2O)2]12- (I), ([(B-α-PW9O34)2Cd4(H2O)2]10- (II), [(B-α-PW9O34)2Mn4(H2O)2]10- (III), [(B-α-PW9O34)2Co4(H2O)2]10- (IV), [(B-α-PW9O34)2Zn4(H2O)2]10- (V), [(ZnW9O34)2WZn3(H2O)2]12- (VI), [(ZnW9O34)2WCd3(H2O)2]12- (VII), and [(B-α-PW9O34)2Zn2Cd2(H2O)2]10- (VIII)) compounds, and Propidium iodide (PI) staining was used to examine the apoptotic effect of I, III, V, and VI POMs.
Experimental
Materials and apparatus
Without any additional purification, all analytical-grade reagents were used just as they were obtained from commercial sources. [A—SiW9O34]10- was synthesized according to the literature and characterized by IR spectra [50]. FT-IR spectroscopy was used to characterize the S2-S8 compounds after they were synthesized by the published procedures [10, 51–54]. A KBr pellet was used to record FT-IR spectra on a Shimadzu 8400S FT-IR spectrophotometer within the 4000–400 cm–1 range. The NMR spectrum was recorded on a Brucker BRX–300 AVANCE Spectrometer. The 29Si and 113Cd nuclei resonance frequencies are 99.36 and 110.92 MHz, respectively. Chemical shifts for 29Si and 113Cd NMR spectra were externally referenced relative to SiMe4 and 0.1 M cadmium perchlorate, respectively. Elemental analyses were carried out in an Integra XL Inductively Coupled Plasma Spectrometer. All electrochemical analyses were performed using deionized water. The solutions were kept in N2 atmosphere throughout the experiment and fully deaerated for at least 20 min using pure N2 bubbles. The Metrohm 797VA computrace polarographic analyzer was used for the electrochemical setup. There was a standard three-electrode setup. Glassy carbon (GC) served as the working electrode. An Ag/AgCl (3 M KCl) electrode was the reference electrode, and a platinum electrode was the counter electrode. Every potential was recorded and measured with the Ag/AgCl electrode. Every voltammetric test was conducted at room temperature. For electrochemical investigations, 0.5 M buffer solutions of NaOAc/HOAc (pH = 4.6) and Na2SO4/H2SO4 (pH = 1.6, 2.6, and 3.6) were prepared by combining different amounts of related acids and salts.
Synthesis of compound (NH4)10Na2[(B-α-SiW9O34)2Cd4 (H2O)2]·22H2O (I)
I was synthesized as a new method in comparison to published work [55]. A 10.0 g (3 mmol) of [A—SiW9O34]10- was added in small portions with stirring to a solution of 3.08 g (10 mmol) of Cd(NO3)2·4H2O in 100 ml of distilled water. This suspension was heated at 50–60 ºC for 3 h and then cooled to room temperature. All insoluble materials were filtered, and 15 g of potassium chloride was added to the solution. The mixture was stirred for 15 minutes and filtered off. This solid was recrystallized from 60 ºC H2O and dried under vacuum. (Yield 1.16g, 13.6%). Elemental analysis for K12[Si2W18Cd4O68(H2O)2]·18H2O: Calcd: K, 8.18; Si, 0.98; W, 57.73; Cd, 7.84; H2O, 5.65 %. Found: K, 8.01; Si, 0.91; W, 57.61; Cd, 7.73; H2O, 5.51%. Using NH4Cl instead of KCl throughout the synthesis process, the crystals of ammonium salt suitable for crystallographic analysis were created. 29Si NMR in D2O: δ –84.9 ppm. 113Cd NMR in D2O: δ 53.40, and 87.75 ppm. Selected FT-IR bands in 1400–700 cm–1 (KBr pellets): 987 (s), 949 (s), 893 (s), 846 (s), and 744 (s) cm–1.
Preparation of I-CPE
To prepare I-CPE, 0.03 g of I powder and 0.02 g of graphite were combined and ground into a homogeneous mixture using an agate mortar and pestle. Subsequently, a glass rod was used to agitate the solid mixture after 0.4 mL of paraffin was added. A glass tube with a diameter of 0.8 mm was filled with the homogeneous mixture, and a copper wire was used to make an electrical connection to the electrode’s back. By rubbing the electrode surface on the soft paper, the electrode’s external surface was cleaned.
Single crystal structure description of I
A transparent I plate with dimensions of 0.20 x 0.15 x 0.03 mm3 was employed for the X-ray work. Intensity measurements were taken at 110 K on a Bruker SMART 1000 CCD single crystal diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073Å). A total of 12220 independent reflections (2θmax = 60.12˚ and Rint = 0.0996) were recorded, of which 8862 reflections were considered [I > 2σ(I)]. The final cycle of refinement converged at R = 0.0658 and of RW = 0.1578 [I > 2σ(I)]. The intensities were corrected for Lorentz-polarization effects and the SADABS program [53] was applied for absorption correction. The structure was refined with SHELXL–97 [51] by full-matrix least-squares on F2. The weighting scheme was w = 1/[σ2(F02) + (0.1056P) 2] where P = (F02 + 2Fc2)/3.
Cell lines and cell cultures
Human breast cancer cell line (MCF–7), murine melanoma cell line (B16F10), and human prostate cancer cell lines (PC3 and DU145) were purchased from the Pasteur Institute (Tehran, Iran) and were kept at 37°C in a 90% humidified environment with 5% CO2. Cell lines were cultivated in 5% (v/v) fetal calf serum (FCS), 100 units/mL penicillin, and 100 μM streptomycin in Roswell Park Memorial Institute medium (RPMI). For each concentration and time course study, there was a control sample which remained untreated and received an equal volume of medium.
Cell Proliferation Assay
Utilizing the reduction power of living cells, the active ingredient in alamarBlue®, resazurin, converts to resorufin, serving as a cell viability indicator. The reduction happens in the living cytosol of the cells. Resorufin is a reddish-colored, highly fluorescent molecule, while resazurin is a blue, practically non-fluorescent, non-toxic, cell-permeable substance. Viable cells constantly transform resazurin into resorufin, which intensifies the overall fluorescence and color of the media around the cells [56]. Around 5×103 MCF–7, B16F10, PC3, and DU145 cells were cultured in each well of 96-microwell plate and incubated with different concentrations of each extract sandwich-type POMs. As directed by the manufacturer, alamarBlue® (10 mg/Dl in PBS) was introduced to each well after a 48-hour incubation period. The cell viability was assessed after 4 hours in culture using a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA) to measure the absorbance at 570 and 600 nm.
PI Staining
PI staining of treated cells was used to identify apoptotic cells and flow cytometry was used to find the so-called sub-G1 peak [57, 58]. Little particles of DNA are produced during DNA fragmentation and can be eluted after being incubated in a hypotonic phosphate-citrate buffer. Cells lacking DNA will appear to the left of the G1 peak and will absorb less color when tagged with a quantitative DNA-binding dye like PI. Summarily, 5×104 MCF–7 cells were placed in each well of a 24-well plate and subjected to different concentrations of the S1, S3, S5, and S6 for 48 h. Following the collection of floating and sticking cells, 400 μl of a hypotonic buffer (50 μg/ml PI in 0.1% sodium citrate plus 0.1% Triton X–100) was added, and the cells were incubated at 4°C overnight in the dark. The cells were then subjected to flow cytometric analysis using a FACScan flow cytometer (Partec GmbH, Münster, Germany).
Statistical analysis
Unless otherwise noted, all results are means ± SEM from triplicate tests carried out in parallel. One-way ANOVA was used to examine any statistical differences between the groups, and Bonferroni post hoc tests were then performed. The significance of the difference is denoted by the symbols *P < 0.05, **P < 0.01, and ***P < 0.001, and all comparisons were done in relation to untreated controls.
Results and discussion
Synthesis of I
The new CdII substituted polyoxometalate, I, which can be separated in pure form as its NH4+ and K+ salts, is produced via the reaction of [A—SiW9O34]10- in an aqueous solution with Cd2+ ions. In 2011, Lijuan Chen et al. synthesized silicotungstate sandwich-type dimer K18{[Mn(H2O)3]2[Mn(H2O)2][(B-β-SiW9O33(OH))Mn3(H2O)(B-β-SiW8O30(OH))]2}. 16H2O by [γ-SiW10O36]8- ions as a primary compound. This reaction must involve rotational isomerization of [γ-SiW10O36]8- (γ β lacunary Keggin) [59]. This is an interesting outcome since the formation of I involves the rotational isomerization of A—SiW9O34 to B-α-SiW9O34 followed by cadmium insertion. We found that the [SiW12O40]4- anion was the predominant product when solutions of
I was passed through a strongly acidic ion exchange column (or was maintained under acidic conditions with pH < 1). Some sandwich-type heteropolyanions show this behavior[54]. This homology is an indirect cue that the skeletal arrangement of the I silicotungstate sandwich-type dimer is comparable to a Keggin structure.
X-ray Single-crystal structure analysis of the NH4+ salt
Table 1 lists the structural refinement parameters and crystallographic data. The single crystal analysis shows that the crystalline I is crystallized in the diagonal space group P2(1)/n (No. 14).
Empirical formula | H88Cd4N10Na2O92Si2W18 |
Formula weight | 5561.86 |
Temperature(K) | 110(2) |
Wavelength | 0.71073 |
Space group | P2(1)/n (No. 14) |
Unit cell dimensions | a = 16.2706(18) Å α = 90º b = 13.8262(17) Å β = 113.419(2)º c = 20.307(3) Å γ = 90º |
Volume | 4192.1(9) Å3 |
Z | 2 |
Density(calc) | 4.406 Mg/m3 |
Absorption coefficient | 25.745 mm–1 |
Reflections collected | 49812 |
Independent reflections | 12220 [R(int) = 0.0996] |
Reflections (I > 2σ(I)) | 8862 |
Data / restraints / parameters | 12220 / 0 / 577 |
Goodness of fit on F2 | 0.982 |
Largest diff.peak and hole | 5.001 and –3.474 e.Å–3 |
Final R indices | R(F0)a = 0.0658 Rw(F0)b = 0.1578 |
a R = Σ||F0| - |Fc||/Σ|F0|.
b Rw = [Σw(F02—Fc2)2/Σ(F02)2]1/2.
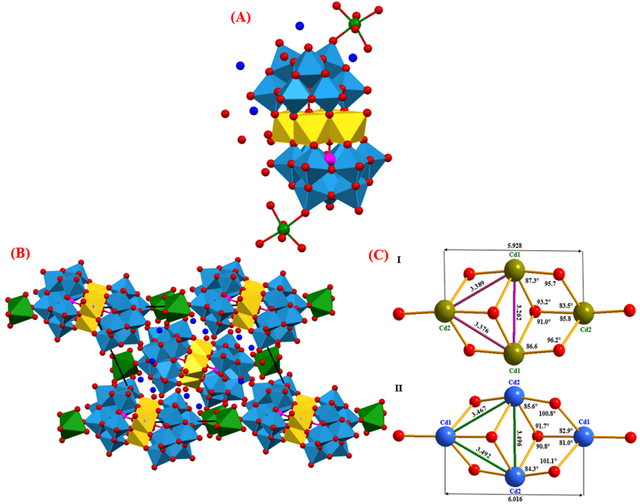
In Figure 1A, the structure of I is displayed. Two (B-α-SiW9O34) ligands are presented in the anion, connected by a Cd4O16 unit made up of four edge-sharing CdO6 octahedra. This structure is the same of [{SiM2W9O34(H2O)}2]12- (M = Mn2+, Cu2+, and Zn2+) and [(B-α-PW9O34)2Cd4 (H2O)2]10- which was previously reported [10, 15]. The unit cell of I is shown in Figure 1B. Two Na+ cations connect with the two terminal oxygen atoms of I. (O34 in each half of the B-α-SiW9O34 unit in I).. The average distance between these Na+ ions and oxygen atoms is 2.426 Å (Table S1, Supporting Information). The Na+ ions can be considered linked to the oxygen by comparing this distance to other bond lengths in I. Figure 1C provides an overview of the relevant angles and distances for the Cd4O16 unit of I and II. As can be seen, the values of bond lengths of Cd cations in the Cd4O16 unit for II are greater than I due to the larger size of the phosphorus atoms.
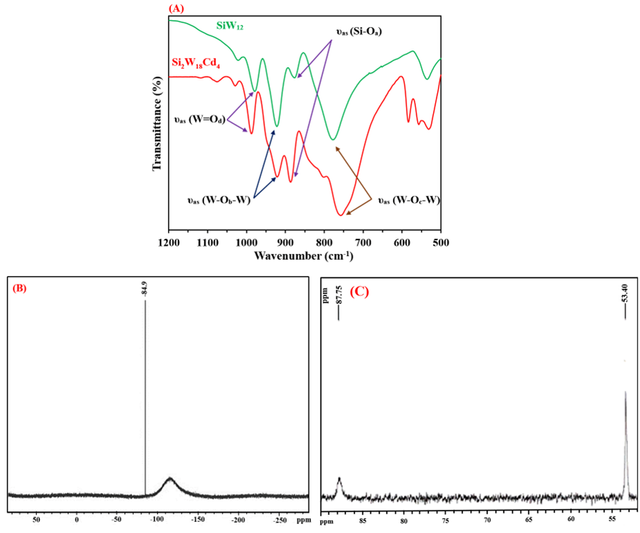
FT-IR, 29Si and 113Cd NMR spectroscopy
The FT-IR spectra of [SiW12O40]4- and I are compared in Figure 2A. Five bands are observed in the FT-IR spectrum of [SiW12O40]4- at 1020, 973, 922, 875, and 775 cm–1. On the other hand, the IR spectrum of I shows six bands at 987, 949 (sh), 920, 893, 802, and 755 cm–1. The broad bands of [SiW12O40]4- at 775 cm–1 split into two bands at 802 and 755 cm–1. It is due to the reduction of symmetry in I. The FT-IR spectra of [PW12O40]3- and [[(B-α-PW9O34)2M4(H2O)2]10- (M = Co2+, Cu2+, and Zn2+) also show this similarity [51]. These bands are related to the SiO4 tetrahedron, W = O (terminal), and W-O-W (corner and edge-sharing WO6 octahedra) [55].
One peak at –84.9 ppm is observed in the 29Si NMR spectrum of I, indicating that there is just one type of silicon present in the dimeric compound (Figure 2B). This pattern shows a single and highly pure product. This peak is assigned to the central SiO4 unit of the silicotungstate [60]. The 29Si NMR chemical shift patterns for some silicotungstate sandwich-type polyoxometalates are shown in Table 2.
compound | δ ( 29Si NMR), ppm | ref. |
α-Si2W18(PhSnOH)3 | –83.2 | [13] |
α-Si2W18(BuSnOH)3 | –82.3 | [13] |
β-Si2W18(PhSnOH)3 | –82.4 | [13] |
β-Si2W18 (ZrOH)3 | –84.2 | [12] |
α-Si2W18SnІІ3 | –85.2 | [13] |
β-Si2W18SnІІ3 | –85.0 | [13] |
Ag2(SiW9O31)2(CH3COO)3 | –83.6 | [17] |
I | –84.9 | This work |
There are two prominent peaks in the 113Cd NMR spectra of I (Figure 2C) at 53.3986 and 87.7500 ppm. This pattern is consistent with single-crystal evidence, indicating that the I includes two forms of cadmium. In I anions, Cd4O14(H2O)2 bridges join two B-α-SiW9O34 units and there are two types of Cd2+ ions in the Cd4O14(H2O)2 fragment. One form, which consists of two Cd atoms, is entirely coordinated by oxygen atoms, whereas the other also has H2O molecules as ligands. It is possible to explain the peak broadening and its multiplicity by the indirect spin-spin interaction of the 29Si and 113Cd nuclei through Cd-O-Si and Cd-O-Cd bonds. Similarly, the 113Cd NMR spectrum of [(B-α-PW9O34)2Cd4 (H2O)2]10- displays two peaks at 26.25 and 52.76 ppm. The difference in values between the 113Cd NMR of the two compounds is related to the more negative charge of I composition compared to [(B-α-PW9O34)2Cd4 (H2O)2]10- compound.
Electrochemical behavior of I
The cyclic voltammogram of I in acetate buffer solution (pH = 3.6 and 4.6) is shown in Figure 3. In pH = 4.6 (Figure 3A), the very strong quasi-reversible redox wave appears in the half-wave potential E1/2 = –0.86 V {E1/2 = (Epa + Epc)/2}. In voltammogram of I at pH = 3.6 (Figure 3B), several quasi-reversible waves can be seen at potentials E1/2 = –0.6-, –0.73, and 0.80V. The oxidation and reduction peaks related to E1/2 = –0.6 V (WVI → WV), –0.73 V (WVI → WV; CdII → Cd), and –0.83 V ( WVI → WV). According to Fig. 3C and the fact that the oxidation-reduction potentials of cadmium and tungsten ions are observed at negative potentials, it can be said that these waves are related to the oxidation-reduction reactions of cadmium and tungsten centers that overlapped with each other. It can also be noted that these waves are pH-dependent.
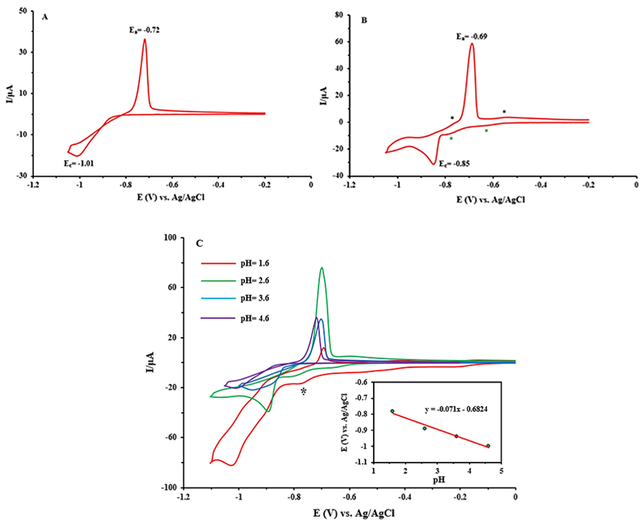
POMs typically undergo protonation in conjunction with reduction, which means that their electrochemical activity is pH-dependent. Figure 3C presents the CV changes of I-CPE as a function of pH. Over the pH range of 1.6–4.6 in buffer solution, wave currents decrease and all wave potentials eventually shift toward negative potential as the pH rises. The Nernst equation explains these observations. The linear relationship between the corresponding redox potential and pH is displayed in the inset of Figure 3C. The wave slope is –71 mV/pH, which is equal to the transfer of approximately two protons per two-electron reduction of tungsten and cadmium centers in comparison with the theoretical value (–59 mV/pH for 2e-/2H+) [61]. The following equation explains this for I:
[Si2WVI18Cd4(H2O)2O68]12- + 2H+ + 2e- → [H2Si2W16VIWV2Cd4(H2O)2O68]12-
Electrocatalysis of NO2- Reduction by I-CPE
The results of the nitrite reduction study have several wider implications, especially for environmental research, public health, and agricultural methods. Water bodies frequently contain nitrite, a pollutant that comes from wastewater or agricultural runoff. These problems can be minimized, and hazardous nitrite can be changed into less dangerous forms by using POMs for nitrite reduction. Many studies have been conducted on the reduction of nitrite ion by Keggin structures, but no reports have been presented on sandwich polyoxometalates. The important point is that Keggin compounds are stable at low pH, but the compound used is stable at high pH [61].
Research on the electrocatalytic characteristics of I-CPE has revealed that the compound possesses appropriate electrocatalytic activities for the reduction of nitrate ions, which are associated with tungsten and cadmium centers. HNO2 can slowly disproportionate under the below reaction:
3HNO2 →NO3- + 2NO + H+ + H2O
To ensure that the influence of this reaction is minimal, experimental data relevant to nitrite catalysis were always obtained from freshly prepared solutions. According to previous work and based on using the differential electrochemical mass spectrometry (DEMS) technique, the reaction of HNO2 or NO2 with the reduced POM produces the gaseous products (NO and N2O). Only NO is detected by the POM’s initial one-electron reduction procedure. However, N2O is produced along with NO when the POM is reduced by two electrons [62].
Because I-CPE should be an excellent candidate for this catalytic impact, we investigated its electrocatalytic activity on nitrite reduction at various pH values. Measurements must be done as soon as nitrite ions are added since a longer waiting period reduces the catalytic effect of POMs. In Figure 4A, the electrocatalytic activities of I-CPE in the reduction of nitrite ions are studied. We find that upon adding NO2-, the reduction current at the carbon paste electrode noticeably increased in the presence of I and the oxidation peak current decreased, while the naked glassy carbon does not show any response to NO2- ion. This behavior shows that nitrite ions can be catalyzed by I [63].
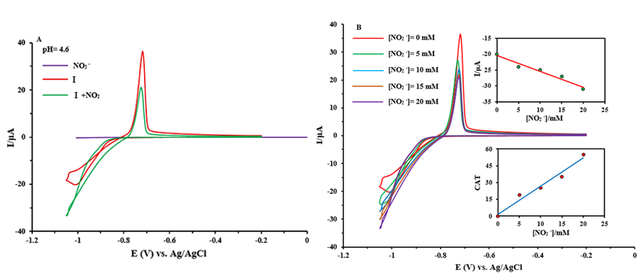
As illustrated in Figure 4B, when the sodium nitrite was increased, the cathodic current increased and the anodic current reduced until the anodic wave disappeared. This behavior suggests that nitrite ions are being reduced electrocatalytically, as would be expected from an electrocatalytic reaction. The catalytic efficiency of POM is defined by CAT = [{IC (POM, NaNO2) - IC(POM)}/IC(POM)] ⁎100 where IC (POM) and IC (POM, NaNO2) are the cathodic peak currents in the absence and the presence of nitrite ion, respectively [64]. The maximum calculated values of CAT for the last wave and highest γ at pH = 4.6 were obtained at 55% for I. This result suggested that I is a good electrocatalyst for NO2− ions.
Cytotoxicity of various POMs
The cytotoxic potential of substances I-VIII was examined on MCF–7, B16F10, PC3, and DU145 cells. The viability percentages of various concentrations of I-VIII POMs are given in Table 3. As demonstrated, POMs VI, VII, V, and III exhibit the lowest viability percentages at a concentration of 500 µg/ml in MCF–7, B16-F10, PC–3, and DU–145 cell lines, respectively. All investigated POMs had lower IC50s than 1 µM in the MCF–7 cell line which were also lower than the doxorubicin (DOX) IC50 value. II-VIII had IC50 values less than 1 µM in the B16F10 cell line. The IC50 for I-III and V was less than 1 µM in the PC3 cell line. Although all of the IC50 values of tested POMs were higher than DOX IC50 value in the B16F10 and PC3 cell lines. In the DU145 cell line, all of the evaluated POMs’ had IC50 levels lower than 1 µM and among them the IC50 values of I and II POMs were lower than DOX IC50 value. Table 4 contains the IC50 values for I-VIII components.
Viability (%) ± SEM in different cell lines | |||||
I | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 47.8 ± (4.6) | 60.4 ± (0.8) | 56.4 ± (5.4) | 27.5** ± (0.9) | |
100 | 33.5 ± (3.9) | 52.6* ± (6.1) | 52.4* ± (1.4) | 17.8** ± (3.9) | |
500 | 25.8* ± (1.2) | 28.6* ± (1.1) | 28.7** ± (2.0) | 9.1** ± (2.8) | |
II | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 44.9 ± (2.0) | 35.9* ± (1.2) | 63.1 ± (1.5) | 30.4** ± (4.2) | |
100 | 33.3* ± (1.7) | 25.2* ± (4.0) | 51.8* ± (1.3) | 20.7** ± (0.8) | |
500 | 30.3* ± (1.6) | 5.7* ± (2.4) | 13.1 ± (19.3) | 12.3*** ± (1.1) | |
III | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 40.1* ± (0.7) | 49.5* ± (1.1) | 57.4* ± (3.6) | 27.2* ± (8.1) | |
100 | 34.8 ± (3.1) | 35.7 ± (9.3) | 27.2* ± (0.7) | 21.2*** ± (2.3) | |
500 | 30.0* ± (1.7) | 13.5* ± (4.2) | 4.7*** ± (7.1) | 4.2** ± (5.1) | |
IV | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 42.0* ± (1.6) | 43.0* ± (1.5) | 84.2 ± (2.0) | 38.9* ± (5.9) | |
100 | 34.5 ± (6.0) | 40.1* ± (0.2) | 65.9* ± (2.5) | 16.1** ± (3.8) | |
500 | 20.3* ± (3.1) | 25.9* ± (6.0) | 51.6** ± (6.9) | 14.4 ± (23.5) | |
V | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 59.0 ± (6.6) | 47.9* ± (1.6) | 47.3* ± (1.1) | 44.6* ± (4.1) | |
100 | 39.3 ± (5.9) | 44.7* ± (1.3) | 26.8** ± (1.4) | 26.7** ± (1.0) | |
500 | 22.1 ± (6.1) | 16.4* ± (2.5) | 3.3* ± (9.7) | 21.7*** ± (0.7) | |
VI | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 16.5 ± (3.5) | 58.3* ± (0.1) | 74.0 ± (1.8) | 48.5** ± (1.6) | |
100 | 8.9 ± (5.0) | 29.3* ± (1.4) | 48.5* ± (2.5) | 41.7* ± (3.9) | |
500 | 2.4* ± (2.8) | 4.9* ± (1.6) | 22.0* ± (4.6) | 25.8 ± (17.9) | |
VII | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 38.3 ± (5.5) | 65.9 ± (3.6) | 67.6 ± (3.3) | 40.7* ± (7.7) | |
100 | 20.6 ± (6.6) | 37.7** ± (6.9) | 49.2* ± (1.2) | 30.2* ± (5.7) | |
500 | 6.6 ± (5.2) | 4.7*** ± (5.4) | 26.9 ± (18.5) | 17.1* ± (7.9) | |
VIII | Concentration (µg/ml) | MCF–7 | B16-F10 | PC–3 | DU–145 |
20 | 45.9 ± (5.3) | 59.9* ± (2.1) | 95.3 ± (3.7) | 47.0** ± (2.1) | |
100 | 36.4 ± (4.8) | 27.7* ± (7.1) | 80.5 ± (2.9) | 26.0** ± (2.3) | |
500 | 34.1 ± (4.0) | 7.8** ± (3.1) | 51.8 ± (10.9) | 8.8* ± (12.7) | |
* P value 0.05; ** P value 0.01; *** P value 0.001
IC50 (µM) | |||||||||
I | II | III | IV | V | VI | VII | VIII | DOX (reference) | |
MCF–7 | 0.509 | 0.373 | 0.199 | 0.411 | 0.819 | 0.152 | 0.404 | 0.379 | 1.189 [65] |
B16-F10 | 1.005 | 0.369 | 0.623 | 0.401 | 0.628 | 0.695 | 0.817 | 0.725 | 0.023 [66] |
PC–3 | 0.917 | 0.934 | 0.703 | 2.100 | 0.575 | 1.096 | 1.050 | 2.041 | 0.240 [67] |
DU–145 | 0.205 | 0.213 | 0.251 | 0.404 | 0.458 | 0.576 | 0.375 | 0.551 | 0.250 [68] |
Apoptosis induction by I, III, V, and VI
Using PI staining test and flow cytometry, apoptosis in MCF–7 cell lines was identified. For 48 hours, cells were treated with different concentrations of I, III, V, and VI (25 µg/ml and 50 µg/ml). The induction of apoptosis in treated cells was demonstrated by the comparison of the Sub-G1 peak in flow cytometry histograms between treated cells and untreated control cells ( = Sub-G1 peak). The flow cytometry histograms are shown in Figure 11.
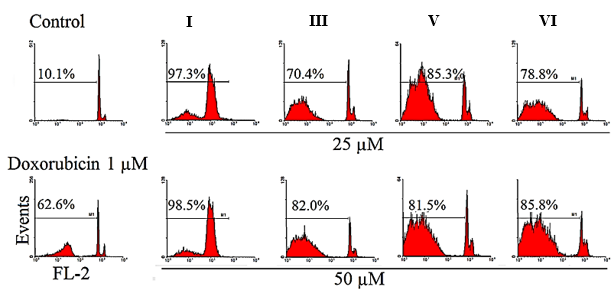
Apoptosis can be induced through multiple pathways, including effects on the cell cycle, protein expression, mitochondrial activity, reactive oxygen species (ROS) generation, and overall cell viability [69].
In 2013, Wang and colleagues investigated the anticancer effects of the dimanganese(II)-containing 20-tungsto–2-bismuthate (III)-based POM-dimer salt (Himi)2Na4[(Mn(H2O)3)2(W(OH)2)2 (BiW9O33)2]·30H2O ({MnBi}) on gastric cancer SGC–7901 cells. Their findings showed that {MnBi} inhibited cell proliferation and induced apoptosis in a dose-dependent manner. Moreover, treatment with {MnBi} led to a reduction in the expression of bcl–2 and nuclear factor-κB p65 proteins, which are associated with cell survival and inflammation [70]. In 2014, Leon et al. found that [Cu4(H2O)2(PW9O34)2]·20H2O triggers apoptosis in MG–63 osteosarcoma cells by increasing ROS levels, lowering the GSH/GSSG ratio, and inducing G2 phase cell cycle arrest in a dose-dependent manner (25–100 µmol/L). Notably, it demonstrated a stronger antiproliferative effect on MG–63 cells (IC50 = 22 µmol/L) compared to normal osteoblasts (IC50 = 92 µmol/L) and outperformed cisplatin in its effectiveness [71]. In 2018, Zhao et al. showed that another sandwich-type POM ({Na0.7Ni5.3(H2O)2(imi)2-(Himi)(SbW9O33)2}6- (imi = imidazole)) was extremely cytotoxic against gastric cancer cells (AGS and BGC–823) by interrupting the cell cycle in the S-phase and triggering cell apoptosis [32]. In 2018, Luo et al. revealed that Na2K0.5Sm0.5[Sm(H2O)8][K3Cu2WO(H2O)10][B-α-AsW9O33]2·14H2O may cause HepG2 cells and HCT–116 cells to undergo autophagy through the participation of lysosomes and apoptosis through the activation of caspase–3 in both cases [72]. In 2019, Wang et al. evaluated the anti-tumor activity of [Na(H2O)4][{Na3(H2O)5}{Mn3(bpp)3} (SbW9O33)2}]·8H3O (bpp = 1,3-bis(4-pyridyl) propane) in vitro and in vivo. They observed that this sandwich-type POM could inhibit the proliferation of human cancer cell lines, including SGC–7901, HT–29, HepG2, Hela, U2OS, SaoS2, and HMC cells, and also suppress the development of tumor xenograft in mice, induce cell apoptosis, and release cytochrome c [73]. Consequently, the substances in this study may demonstrate their cytotoxicity activity in cancer cell lines by inducing apoptosis.
Conclusion
In this work, we have successfully synthesized the (NH4)10Na2[(SiW9O34)2Cd4 (H2O)2]·22H2O (I) in a simple method. This compound was characterized by routine techniques and then its structure was confirmed by single-crystal X-ray diffraction. Single-crystal X-ray diffraction, 29Si NMR, and 113Cd NMR spectroscopy prove the presence of silicon and cadmium atoms in the I compound. The redox wave of the cadmium and tungstate centers are pH-dependent. Over the pH range of 1.6 - 4.6 in buffer solution, the redox wave moves in the negative direction as the pH increases. The study of electrochemical and electrocatalytic properties exhibits that I have good electrocatalytic activity in the reduction of NO2ˉ.
According to the results obtained from this study, it seems that I-VIII POMs have shown considerable cytotoxicity on MCF–7, B16F10, DU–145, and PC3 cell lines. I, III, V, and VI compounds were investigated for their ability to induce apoptosis. It was discovered that these compounds significantly affected apoptosis induction and could be employed as a potential chemotherapeutic drug.
Appendix A
Crystallographic data for structural analysis I have been deposited with the Cambridge Crystallographic Data Center, CCDC No. 859177. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Tel: +44 (0)1223 762911; e-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
Statements and Declarations
Authors' contributions
All authors contributed to data analysis, drafting, and revising of the paper and agreed to be responsible for all theaspects of this work.
Competing Interests
The authors declare no competing interest.
Ethics Approval
Not applicable.
Data Availability
Data will be made available on request
Funding
This paper received no external funding.
Authors’ Information
Zahra Tayarani-Najaran—Department of Pharmacodynamics and Toxicology, School of Pharmacy; Mashhad University of Medical Sciences, Mashhad, Iran.
Nasrin Mohajeri—Samen Pharmaceutical Company, Mashhad, Iran.
Aidin Mohammadi Zonouz—Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran.
Farrokhzad Mohammadi Zonoz—Department of Chemistry, Faculty of Science, Hakim Sabzevari University, Sabzevar, Iran.
Mehdi Baghayeri—Faculty of Chemistry, Silesian University of Technology, 44–100 Gliwice, Poland; Research Core of Advance Photo-electro Materials (APEM), Faculty of Science, Hakim Sabzevari University, Iran.



.tif)
 - Copy copy.png)


