Visual Abstract
Abstract
Colorectal cancer (CRC) is a major global health concern, representing 10% of cancer diagnoses and about 9% of cancer-related deaths worldwide. It is the third most common cancer globally and ranks fourth in cancer-related mortality. MXenes (M is transition metal and X represents carbon or nitrogen), a versatile group of two-dimensional (2D) nanomaterials including transition metal carbides/carbonitrides/nitrides, are notable for their distinctive physicochemical characteristics, including varied surface chemistry, extensive surface area, and biocompatibility. They have found utility in various biomedical fields like drug delivery, biosensing, and cancer therapy. However, MXenes encounter challenges in biomedical fields because of poor physiological stability and decomposition. These challenges can be surmounted by creating multifunctional MXene composites with other materials. Despite extensive research on MXenes in cancer therapy, their application in CRC treatment remains underexplored, necessitating further investigation. Therefore, developing MXene-based platforms for various innovative approaches is a promising area of research. These approaches aim to leverage MXenes' distinctive properties for enhancing treatment strategies, including drug delivery, hyperthermia, and photothermal therapy. In this review, MXene preparation methods, CRC treatment methods, information about this type of cancer therapy using MXene-based systems, and future perspectives and opportunities are discussed.
Introduction
CRC is a significant global health concern. In addition to genetic factors, which are the most important risk factors, higher rates of smoking, sedentary lifestyles, consumption of sugary drinks, processed meat, and alcohol contribute to the increasing incidence of CRC [1, 2]. In 2018, there were 1.8 million new cases of CRC, and 881,000 deaths related to the disease. It accounted for approximately 10% of all new cancer cases and cancer-related deaths globally [3]. Projections suggest that the number of new CRC cases may rise to nearly 2.5 million by 2035, highlighting the growing impact of this disease on public health [4, 5]. Surgical removal is the preferred method, but despite screening programs, a significant number of cases are diagnosed late (at a metastatic stage( [6, 7]. For those who cannot undergo surgery, the goal is to shrink and control tumor growth using radiotherapy and chemotherapy. In some cases, radiotherapy and/or chemotherapy may be used before or after surgery to reduce and stabilize the tumor [8, 9]. Furthermore, severe pain, complex procedures, wound infection, and prolonged recovery time are other major disadvantages of surgery [10, 11]. One of the best ways to address these challenges is smart drug delivery systems [12]. 2D materials with tunable structures have generated significant excitement in the biomedical field because of their magnetic, photonic, and electronic properties [13].
The introduction of 2D materials sparked considerable progress in the fields of biomedical applications and disease therapy [14–16]. The distinctive characteristics of 2D nanomaterials came to the forefront following their recognition with the 2010 Nobel Prize [17, 18]. These nanomaterials outperformed a single-layered material such as graphene, offering improved attributes, including enhanced performance, flexibility, and greater compatibility [19]. Additionally, their ultrathin profile and unique 2D structure endowed them with distinct physical, chemical, and electronic properties compared to similar materials [20]. MXenes, a subgroup of 2D materials, contain transition metal carbides, nitrides, and carbonitrides. The name “MXenes” is derived from creating these 2D layered materials, where an ‘A’ layer is etched from MAX phases. The addition of the ‘ene’ suffix is meant to underscore their resemblance to graphene [21]. MXenes have a chemical structure described by the formula Mn+1XnTx. Here, ‘M’ stands for an early transition metal (including elements like Ti, Sc, Zr, V, and others.(, ‘X’ encompasses carbon, nitrogen, and, more recently, oxygen, and ‘T’ represents surface terminating functionalities. (such as –F and –OH, and -Cl). The variable ’n’ in the Mn+1XnTx formula corresponds to the number of M and X layers in a 2D MXene flake, and it can range from 1 to 3.
Synthesis of MXenes
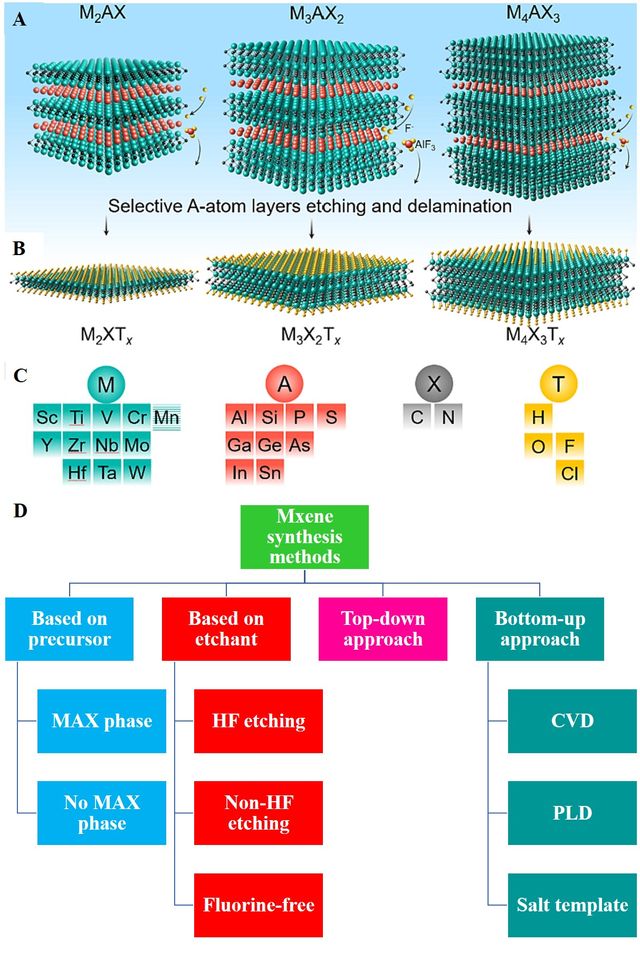
Preparation techniques for MXenes, a novel category of 2D nanomaterials, have undergone significant development since the initial discovery of Ti3C2Tx in 2011 by the selective etching (usually by HF) of the MAX precursor Ti3AlC2 [15]. MXenes are generally derived by removing the A layers from their layered precursor MAX phases through etching. Exfoliation follows the etching stage and depends on the etching methods [22]. Over the past years, there has been rapid development in the synthetic methods for MXenes, enabling the creation of an increasingly diverse range of MXene species. Broadly, the methods are categorized into two primary approaches: a top-down process involves direct exfoliation of multilayer bulk parent materials while preserving their original structure. Different driving forces are utilized to eliminate van der Waals interactions among the stacked layers of these bulk materials, such as chemical and mechanical exfoliation techniques. Figure 1 illustrates the top-down synthesis of MXenes, which involves the selective removal of A layers from MAX phases [23]. A bottom-up approach, in which 2D ordered structures are cultivated from small inorganic and organic molecules. This approach offers distinct benefits, allowing for precise adjustment of size distributions, geometric shapes, and surface terminations of MXenes. In contrast to top-down methods, there has been limited research on the bottom-up synthesis of MXenes, possibly because of the complex nature of MXenes structure. Furthermore, the reported methods for the synthesis of MXenes can be categorized as follows [24].
The properties of MXenes
As a newly emerging class of 2D-nanomaterials, MXenes exhibit distinct characteristics when compared to other materials. These properties include excellent electronic capabilities, remarkable optical qualities, exceptional magnetic attributes, superior hydrophilicity, and flexible mechanical properties [25,26]. Certain MXene properties significantly contribute to the development of functional platforms for cancer therapy. These characteristics will be summarized in the subsequent sections:
Mechanical Features
The mechanical attributes of MXenes are notably influenced by their surface terminations. MXenes with oxygen (O) terminations exhibit significant stiffness compared to those with fluorine (F) or hydroxyl (OH) terminations, owing to the stronger interaction between the surface M atoms and oxygen [27–29]. Importantly, surface functionalization can decrease Young’s modulus, expand critical strains to enhance mechanical properties, and heighten overall flexibility [30]. To enhance mechanical characteristics, such as toughness, compressive strength, tensile strength, and overall flexibility, MXene-based composites can be produced by incorporating diverse polymeric materials [31, 32]. For instance, the mechanical properties of Ti3C2 consistently improved when it was combined with various polymers, such as polyethylene, polyacrylamide, and polyvinyl alcohol [33].
High Surface Area
The large surface area and nanostructure of MXenes enable effective drug and targeting component loading, facilitating specific cancer cell treatment with minimal impact on normal cells. The etching process of MXenes leads to the exfoliation of layers that significantly increases the specific surface area. This specific surface area can be additionally expanded by continuing the layering process to achieve a monolayer structure. [34–36]. Generally, factors like etching conditions, temperature, intercalation agent, and reaction time influence MXenes’ morphology. For example, higher etching temperatures can accelerate the process but may compromise structural integrity, while prolonged etching times can increase layer spacing, though excessive time may result in layer restacking.
Biocompatibility and Hydrophilicity
The hydrophilicity of MXenes is because of their oxygen and hydroxyl groups. Their hydrophilicity is closely linked to biocompatibility, higher hydrophilicity typically indicates improved biocompatibility [37]. MXenes’ hydrophilicity and high conductivity enhance their interaction with polymer matrices, benefiting their use in composite materials [38]. Biocompatibility in MXenes is crucial for biomedical applications. Numerous studies have assessed their biocompatibility, including in-vitro tests like the MTT assay. The study demonstrated that some Ti-based MXenes have been identified as biocompatible [39]. For instance, the research reported by Lin and colleagues involved testing soybean phospholipid-Ti3C2‘s effects on 4T1-bearing tumor models with different doses for a month, showing no significant behavioral or weight changes [40]. Additionally, studies have explored MXenes’ biocompatibility against various immune cell subsets, demonstrating their mild and non-toxic nature [41]. Furthermore, their hydrophilic behavior facilitates surface modification and enhances antibacterial performance, potentially aiding in bacterial eradication by direct contact interactions [42]. MXenes, composed of transition metals and biologically relevant elements like nitrogen and carbon, exhibit tunable biocompatibility and potential for biodegradation (their biocompatibility is dose- and modification-dependent). Thanks to the relatively lower cytotoxicity of Ti [43], titanium-based MXenes are widely used in biomedical applications such as CRC treatment [44]. Recently, due to the suitable biocompatibility, non-titanium MXenes such as Mo2CTx, Ta4C3Tx, and Nb2CTx were used in biomedical applications [45]. Controlling the quantity of MXenes is vital to prevent significant cytotoxicity. One approach involves modifying the surface features by hybridization or combination of MXenes with biopolymers, including chitosan, silk fibroin [46], collagen [47], and polydopamine [48], effectively reducing cytotoxicity. The time, dose, size, and oxidative stress are additional factors affecting MXene’s toxicity. Lower MXenes concentrations generally result in minimal cytotoxicity [49]. Notably, studies have shown that the cytotoxicity of Ti3C2 MXenes is time- and dose-dependent, with MXenes causing damage by generating intracellular reactive oxygen species (ROS), impacting proteins and DNA, and leading to cell death [50]. Thus, optimizing MXenes’ parameters is a key strategy for enhancing their biocompatibility.
Versatile Modification for Fabrication of Functional Platforms
Due to the preparation process, numerous functional groups (oxygen, fluorine, or hydroxyl) are often present on the material’s surface. The MXene combines the metal conductivity of transition metal carbides/nitrides with the hydrophilicity of its surface functional groups, offering the potential for straightforward modifications to enhance its specific properties [26, 36]. Some challenges, like toxicity, biocompatibility, and oxidative deterioration in aqueous media, should be addressed for the wider use of MXene-based systems in CRC treatment [21, 51]. Surface modification strategies, such as polymer integration or functional group engineering, effectively address these challenges. Furthermore, the surface modification of MXenes enables their facile integration with various kinds of materials through electrostatic interaction, intermolecular forces such as hydrogen bonding, and van der Waals force. These materials can include polymers (like PEG (polyethylene glycol), PDA(polydopamine), hyaluronic acid, soybean phospholipids [40], metal/metal oxide nanoparticles (such as Au nanoclusters [52], ZnO [53], Mn3O4, MIONPs (magnetic iron oxide nanoparticles)) [54, 55], and antioxidants (e.g., dopamine and sodium ascorbate), enzyme-like materials (e.g., glucose oxidase and chloroperoxidase), carbon-based materials (g-C3N4 (graphitic carbon nitride) [56, 57], mesoporous silica materials [58]. This approach results in the fabrication of multifunctional MXene-based anticancer platforms with advanced therapeutic capabilities, allowing for combined photo and chemodynamic therapy and multimodal imaging features, surpassing the capabilities of individual MXene [21].
The application of MXene-based platforms in cancer treatment
In the past few years, MXene-based platforms have gained significant attention in the biomedical field, particularly in cancer theranostics, which includes cancer diagnosis and therapy. This is attributed to their outstanding electronic, structural, and chemical features. These features include a high specific surface area for effective modification/functionalization, low bandgap with strong light absorption in the near-infrared region (NIR), hydrophilic surfaces, numerous active sites for drug incorporation, and excellent biocompatibility. Considering these distinctive properties and highly tunable compositions, MXene-based materials can be utilized as versatile tools for cancer diagnosis including i) disease detection through biosensors, ii) lesion localization through bio-imaging, and cancer therapy which includes i) drug delivery ii) photodynamic therapy (PDT), iii) photothermal therapy (PTT), and iv) sonodynamic therapy (SDT), and v)combination therapy [36].
MXene-based platforms in cancer diagnosis
Biosensors
Biosensors are tools for detecting biological materials and converting their concentrations to electrical signals and have gained significance in environmental monitoring, anticancer drug screening, clinical diagnosis, and more. Recent studies highlight that MXenes, with their remarkable surface area and versatile modifiability, have been employed to detect a wide range of analytes, including biomarkers, glucose, drugs, and neurotransmitters. The unique electrochemical properties of MXene-based platforms play a vital role in cancer diagnostics. Biomarkers are biomolecules present in an organism's blood or bodily fluids, undergoing subtle changes during disease conditions and being detected as a signal of disease. The detection of biomarkers in the preliminary stages can help control the rising incidence of cancer and decrease mortality rates. Notable cancer-related biomarkers include Epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), Epithelial cell adhesion molecule (EpCAM), mucin 1, and carcinoembryonic antigen (CEA) [36, 59].
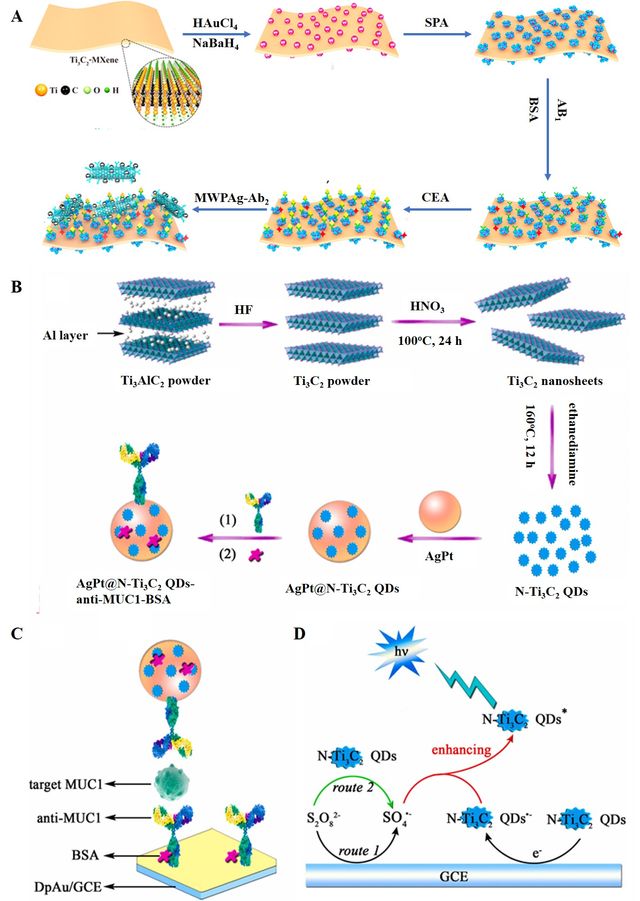
CEA is a widely used tumor biomarker for the screening, diagnosis, and prognosis assessment of diverse gastrointestinal tumors, for instance, colorectal, pancreatic, esophageal, and gastric cancers [60]. Detecting CEA rapidly and sensitively is crucial for cancer diagnosis and treatment. Serum CEA levels are normally below 5.0 ng. mL-1 in healthy adults but increases rapidly in cancerous conditions. Surface plasmon resonance (SPR) is a prominent label-free technique for sensitive and selective bio-affinity assays, offering rapid detection and miniaturization for analyzing various targets. Wu et al. prepared several Ti3C2-based nanocomposites as ultrasensitive SPR biosensing platforms to improve the detection of CEA [61]. First, they prepared Ti3C2-MXene nanosheets by exfoliating the Ti3AlC2 precursor using concentrated HF and then decorated them with gold nanoparticles (AuNPs). The resulting Ti3C2-MXene/AuNPs were subsequently modified with staphylococcal protein A (SPA) to orient and immobilize the monoclonal anti-CEA antibody. This immobilized antibody was utilized to capture and detect the CEA. Afterward, hybrids of multi-walled carbon nanotube-polydopamine-AgNPs (MWCNTs-PDA-AgNPs) were combined with polyclonal antibodies to enhance the response signal, resulting in significantly improved detection sensitivity with a very low detection limit (LOD) of 0.07 fM. In another study, for the detection of mucin 1 (MUC1), nitrogen-doped titanium carbide quantum dots (N-Ti3C2 QDs)-based electrochemiluminescence immunosensor (ECL) were designed (Figure 2). Thanks to the N-Ti3C2 QDs’ ability to promote the reduction of S2O82- which was a co-reactant and responsible for the generation of sulfate radicals, ECL signal enhancement has occurred, which led to low LOD of 0.31 fg.mL-1 [62].
MicroRNAs (miRNAs), consisting of 18-25 nucleotides, are a subset of non-coding RNAs that play a crucial role in many biological activities, such as gene regulation, cell proliferation, apoptosis, energy metabolism, and tumorigenesis [63]. They were initially identified as cancer biomarkers in 2008 by Lawrie et al. Furthermore, the overexpression of miRNAs serves as a biomarker for several diseases, including cardiovascular disorders, viral infection, neurological disorders, diabetes, and different types of cancers [64]. In another research work, Sun and co-workers developed a photoelectrochemical (PEC) biosensor based on MoS2@Ti3C2 nanoplatform for the ultrasensitive detection of cancer biomarker, colorectal cancer (CRC)-related miRNAs (miR-92a-3p) [65]. MoS2 efficiently converts light into electricity due to its outstanding UV-Vis photoelectric conversion efficiency. The incorporation of Ti3C2, known for its excellent electron-transfer capability, greatly improves the photocurrent response in the resulting PEC biosensor. Furthermore, by electrodepositing Au NPs onto the MoS2@Ti3C2 nanocomposite surface, the photocurrent is further improved. The fabricated MXene-based-PEC biosensor, MoS2@Ti3C2@Au NPs, was evaluated for detecting CRC-related exosomal miRNA. Its clinical applicability was confirmed by measuring miR-92a-3p concentrations in both CRC patients and healthy controls. It exhibited a wide linear detection in a range of 1 fM-100 nM with a low LOD of 0.07 fM.
Bio-imaging
MXenes' remarkable NIR light absorption makes them ideal for in vivo photoacoustic (PA) imaging. Furthermore, their distinctive ferromagnetism is well-suited for magnetic resonance (MR) imaging. Additionally, their quantum size effect is highly suitable for photoluminescence (PL) cell imaging, offering substantial elemental contrast [36].
MXene-based platforms in cancer therapy
Drug Delivery
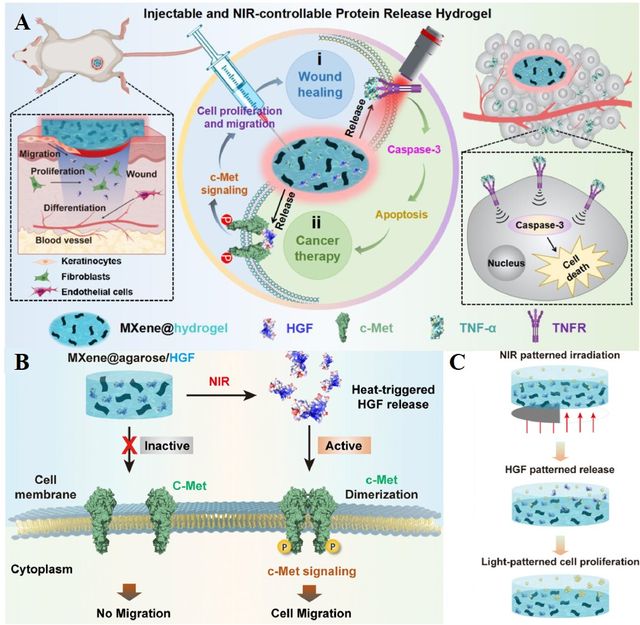
Wang et al. constructed an injectable and NIR light-responsive MXene@hydrogel/protein platform [66]. This photo-therapeutic platform comprised Ti3C2 MXene as a photothermal active agent, agarose, and therapeutic protein. The Ti3C2 MXene, when incorporated with protein drugs in the hydrogel matrix, facilitates the controlled release of therapeutic proteins. (Indeed, MXene transforms NIR light into heat, triggering a reversible phase shift in the matrix to release preloaded protein drugs.) This capability allows for the modulation of receptor-mediated signaling to achieve customized cellular responses. They confirmed the effectiveness of the Ti3C2-based platform for anticancer therapy by loading tumor necrotic factor-α (TNF-α) as a protein-based drug into this platform (MXene@agarose/TNF-α) via in-vitro assessment using the colorectal cancer cell lines (HCT116). This structure allows for NIR light-controllable induction of pro-apoptotic signaling in tumor spheroids. TNF-α, a crucial cytokine primarily generated by macrophages, is employed in cancer therapy due to its ability to trigger the apoptosis of tumor cells through its specific receptor. The therapeutic efficacy of the NIR light-responsive MXene@agarose/TNF-α system was evaluated through an in vivo study using nude mice carrying HCT116 tumors. Tumor size was monitored continuously for two weeks. The reported result indicated that MXene@agarose/TNF-α allows for NIR light-controlled pro-apoptotic signaling, effectively inhibiting tumor progression. Overall, the injectable, NIR light-controllable MXene-based hydrogel offers a robust therapeutic platform for the controlled release of protein-based drugs in deep tissues, thereby enhancing the precision and effectiveness of cancer therapy (Figure 3).
Photothermal Therapy (PTT)
Significant prevalence and mortality of cancer resulted in prompted global efforts to advance precise diagnostic techniques and effectual anticancer approaches. Traditional treatments such as surgery, chemotherapy, and radiotherapy, are generally utilized in clinical treatments, but they face major challenges such as subsequent side effects and the risk of ineffectiveness [67]. Over the years, PTT has emerged as a minimally invasive treatment method with fewer side effects. A PTT treatment method employs materials with strong NIR absorption, which are injected into the body to damage cancer cells by generating intense heat. These agents, injected into the body, accumulate in the tumor site and convert light energy into heat upon exposure to an external light source (typically NIR light). The generated thermal energy through disrupting various cellular structures, such as proteins and membranes, can induce cell death in the tumor region. MXenes and MXene-based platforms have shown excellent potential as photothermal agents for treating deep tumors due to excellent properties such as strong NIR absorption, large surface area, hydrophilic surfaces, multi-functionality, and high photothermal conversion efficiency. For instance, in one study, A Ti3C2-based system was fabricated and showed high photothermal conversion efficiency (34.3%) under 1064 nm radiation. This system induced colorectal cancer cell apoptosis and led to CRC development inhibition [68]. Furthermore, MXenes can function as anticancer drugs carriers and contrast agents in bioimaging, allowing for drug delivery and treatment process monitoring. These combined effects considerably boost and enhance the efficacy and convenience of cancer treatment. The success of PTT is mainly related to factors such as tumor tissue depth, photothermal agent accumulation, photothermal conversion efficiency, optical power density, and photoexcitation duration [58].
Photodynamic Therapy (PDT)
PDT is a non-invasive strategy for cancer treatment, exhibiting impressive performance for clinical application. This strategy started gaining attention in the 1970s and has since shown promise in cancer therapy [69]. The PDT involves three key components: photosensitizer (special drugs), tissue oxygen, and visible light irradiation. These photosensitizing agents are turned on or activated when exposed to a specific wavelength of light irradiation leading to the conversion of 3O2 to a highly reactive substance, mainly 1O2 (singlet oxygen). The produced reactive oxygen species by reacting with biological molecules such as proteins lipids, and nucleic acids eventually lead to cell death via necrosis, apoptosis, or autophagy [70, 71]. 2D nanomaterials such as graphene and MXenes have been employed as photosensitizers offering several advantages over conventional organic photosensitizers These advantages include i) a substantial extinction coefficient, which enhances the energy transfer process, ii), convenience modification: they can be easily modified to reduce toxicity and enhance targeting, iii) High specific surface area: their large specific surface area enables synergistic therapy; they used as carriers for loading of photosensitizers, drugs or other targeting moieties, enabling precise release and cancer cells treatment [72].
Sonodynamic Therapy (SDT)
In SDT approach, ultrasound was used to trigger the production of reactive oxygen species with sonosensitizers, offering targeted cancer cell destruction without harming healthy tissues. Furthermore, the capacity of US waves to penetrate deep tissues enables it possible to treat large tumors or deep-seated or effectively. Hence, SDT emerges as a potential non-invasive therapeutic approach that outperforms traditional PDT [73, 74]. Different metal oxides, organic compounds, black and various hybrid materials have been employed as efficient sonosensitizers in PDT approach for cancer therapy.
Chemodynamic Therapy
Liu and colleagues fabricated Ti3C2 MXene nanosheets with atomic thickness (approximately 100 nm) by introducing a surface functional group, Al(OH)4 as depicted in Figure 3 [75]. This modified Ti3C2 MXene nanosheets is the first reported MXene with the potential to generate ROS when exposed to appropriate wavelength of light suggesting its utility as a photosensitizer for photodynamic therapy approach. The prepared MXene demonstrates remarkable properties, including a high mass extinction coefficient (~28 L/g.), excellent photothermal conversion efficiency (about 58 %), and efficient generation of singlet oxygen (at 808 nm, 10 minutes, 0.8 W/ cm2). Considering the excellent electronic structure (presence of free surface electrons) of modified-Ti3C2 nanosheets, it can synergize with chemotherapy and photodynamic therapy. Therefore, it was employed in combination with doxorubicin) anti-cancer drug and a chemotherapy agent) and hyaluronic acid (an active tumor-targeting agent) through layer-by-layer surface modification technique. The result of several in vitro and in vivo experiments on human colon carcinoma cell line (HCT-116 cells) revealed that obtained multifunctional Ti3C2 based platform displays great biocompatibility, specific accumulation at tumor site, sustained multi-stimuli-responsive drug (DOX) release and synergistic PTT/PDT/chemotherapy. As a result, the surface-modified MXenes serve as a highly effective multifunctional anticancer therapeutic and agent for the destruction of cancerous tissue [66].
Combination Therapy
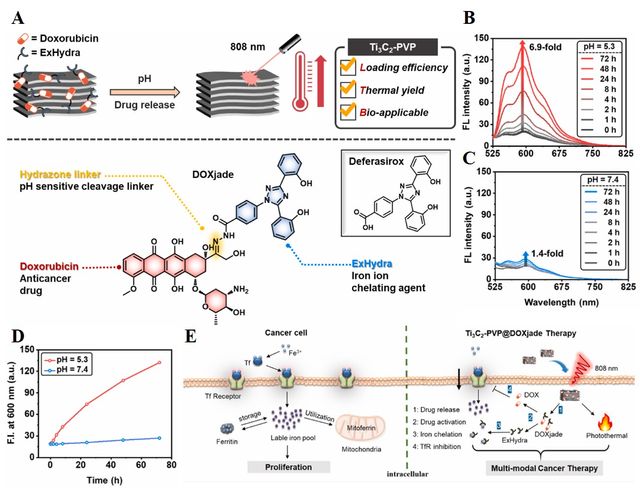
To enhance cancer treatment effectiveness, chemotherapy can be integrated with various therapeutic approaches such as PTT, PDT, SDT, radiotherapy, immunotherapy, and more. As an example, Xu et al. introduced a pH-responsive "dual-therapeutic drug nanomedicine" strategy that combines iron chelation chemotherapy with photothermal therapy [76]. To achieve this, they designed a novel dual-therapeutic conjugate DOXjade combining deferasirox (a clinically approved iron chelator) and DOX (a chemotherapeutic agent that inhibits topoisomerase II activity). DOXjade was then loaded onto 2D Ti3C2 MXene (functionalized with polyvinylpyrrolidone) to serve as both a photothermal generator and a drug nanocarrier for NIR-triggered drug release. The constructed nanoplatform, Ti3C2-PVP@DOXjade, not only allows the iron-chelating and chemotherapeutic capabilities of DOXjade to be selectively (photo-activated) triggered at tumor sites but also provides strong photothermal potential. Upon exposure to NIR light at a wavelength of 808 nm, within the optical-therapeutic window of 650–900 nm, the Ti3C2-PVP@DOXjade platforms exhibited remarkable photothermal conversion efficiencies, reaching up to 40%. They investigated the iron chelation chemo-photothermal performance of Ti3C2-PVP@DOXjade nanoplatforms by in vitro and in vivo experiments on HCT116 cells (human colon cancer cell line). The suggested antitumor mechanisms (Figure 4) showed that activated Ti3C2-PVP@DOXjade causes apoptotic cell death and downregulates the iron transferrin receptor (TfR) due to iron depletion. As illustrated in Figure 4, the main steps in the process include i) DOXjade release from Ti3C2-PVP, ii) hydrolysis of DOXjade into DOX and ExHydra, iii) iron chelation by ExHydra, and iv) inhibition of TfR expression by DOX. In another study, a novel multifunctional system was designed by incorporation of MXene and GelMA [77]. MXene’s photothermal effect and its assistance in the controlled release of 5-Fu and Cangrelor could inhibit tumor cell growth which paved the way for CRC treatment.
Conclusion
This article provides an overview of the utilization of MXene-based platforms for treating colorectal cancer. It emphasizes the global significance of colorectal cancer as a major health issue and the urgent need for improved treatment strategies. MXenes, as versatile 2D nanomaterials, are introduced as promising candidates for the development of multifunctional platforms to address this challenge. This is attributed to their exceptional structural, physical, and chemical properties, including mechanical stability, high surface area, biocompatibility, and the ability to be modified for integration with diverse materials. This review acknowledges the challenges MXenes face in medical and biological applications, particularly their physiological stability and biodegradability, but suggests that these disadvantages can be overcome through the development of multifunctional MXene-based composites. Furthermore, MXenes offer several advantages in colorectal cancer treatment, including strong NIR absorption, high surface area, and biocompatibility, making them highly suitable for multifunctional therapeutic applications. It explores their application in cancer diagnosis and treatment, from biomarker detection to biomedical imaging. Additionally, MXenes have demonstrated promise in cancer therapy, including drug delivery, PTT, and PDT, with the potential for integration into multimodal treatment strategies. These applications are related to the specific properties of MXenes, such as their high photothermal conversion efficiency and their ability to generate ROS when exposed to certain wavelengths of light. Looking forward, the importance of ongoing research in this field and the need for developed approaches to combine the unique properties of MXenes with practical materials to enhance colorectal cancer treatment.
Statements and Declarations
Authors' contributions
The contributions of each author to this study are as follows: Fereshte Hassanzadeh Afruzi and Moein Safarkhani conceptualized the study. Fereshte Hassanzadeh Afruzi and Amirhossein Ojaghi contributed to the literature review and drafted the manuscript. Moein Safarkhani supervised the study.
Competing Interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Data availability
Not applicable.
Funding
This paper received no external funding.
Authors’ Information
Fereshte Hassanzadeh Afruzi—Catalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran.
Amirhossein Ojaghi—Department of Chemistry, Sharif University of Technology, Tehran, Iran.
Moein Safarkhani—School of Chemistry, Damghan University, Damghan, 36716–45667, Iran;



.tif)
 - Copy copy.png)


