Visual Abstract
Abstract
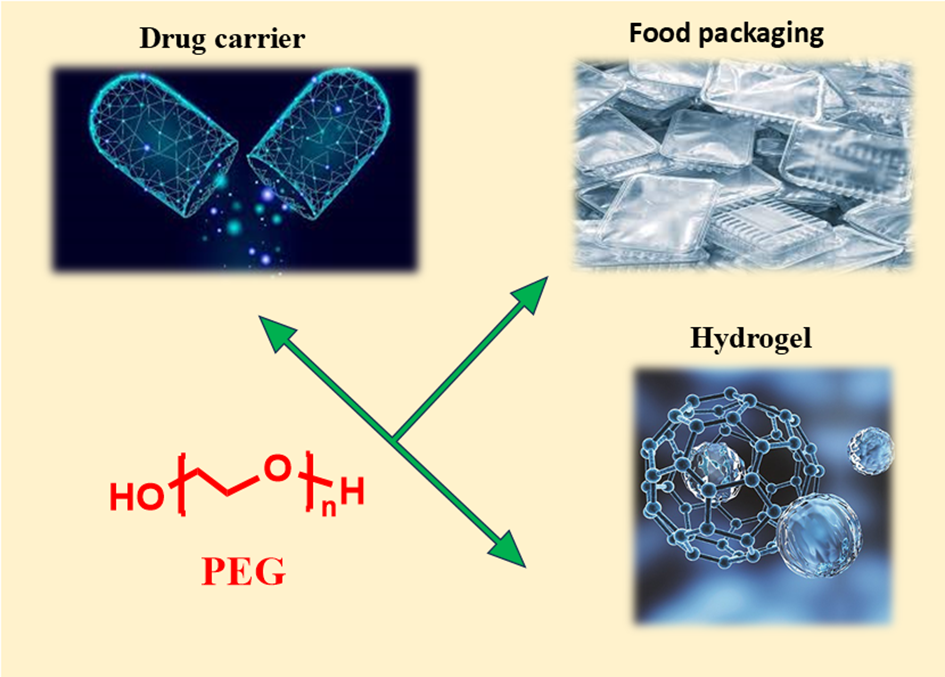
Polyethylene glycol (PEG) has emerged as a versatile polymer with widespread applications in both biomedical and food industries due to its unique physicochemical properties, including excellent biocompatibility, hydrophilicity, and ease of modification. In the biomedical field, PEG-based materials have demonstrated remarkable potential in drug delivery systems, wound healing, and tissue engineering. PEG hydrogels, in particular, have garnered significant attention for their ability to encapsulate and release therapeutic agents in a controlled manner, with stimuli-responsive systems offering tailored drug release mechanisms. In the food industry, PEG-based materials are employed to enhance food quality, extend shelf life, and develop innovative packaging solutions, such as antimicrobial films and nanocomposites. This review comprehensively discusses the recent advancements in PEG-based materials, focusing on their biomedical applications, including drug delivery and wound care, as well as their role in improving food safety and packaging. Additionally, the challenges and limitations associated with PEG, such as limited biodegradability and potential health concerns, are critically evaluated. Finally, future perspectives on the development of next-generation PEG-based materials, with an emphasis on sustainability and clinical translation, are highlighted.
Polyethylene glycol (PEG), PEG hydrogels, Drug delivery, Biocompatibility, Food packaging, Antimicrobial films
Introduction
Polyethylene glycol (PEG) stands as one of the most versatile and widely used polymers in modern science and industry, with applications spanning biomedicine, pharmaceuticals, and the food sector [1–4]. Renowned for its unique physicochemical properties—such as high biocompatibility, water solubility, and tunable molecular structure—PEG has become a cornerstone material in the development of advanced technologies [5–8]. Its ability to adapt to diverse environments through molecular modifications and polymer design has enabled its use in groundbreaking applications, including drug delivery systems, tissue engineering, and food packaging [9–12].
One of PEG’s most distinguishing features is its hydrophilic nature, which allows it to reduce viscosity and enhance solubility in complex formulations [13–15]. This property is particularly valuable in industries where precise control over material behavior is critical, such as in pharmaceuticals and food production. Recent advancements in synthetic methodologies have further refined PEG production, enabling the creation of homogeneous and controllable polymer variants that expand its potential for precise applications [16–19]. These innovations have paved the way for PEG’s integration into cutting-edge materials, including hydrogels, nanocomposites, and antimicrobial films [20–23].
In the biomedical field, PEG has emerged as a transformative material, particularly in drug delivery and hydrogel-based technologies [24–26]. PEG-based hydrogels, known for their controlled drug release capabilities and responsiveness to environmental stimuli, have revolutionized treatments for chronic wounds, tissue inflammation, and osteoarthritis [27–29]. For instance, stimuli-responsive PEG hydrogels have enabled targeted drug delivery, minimizing off-target effects and improving therapeutic outcomes [30–32]. These advancements underscore PEG’s potential to address some of the most pressing challenges in modern medicine.
Beyond its biomedical applications, PEG has also made significant strides in the food industry, particularly in packaging and preservation technologies [33–35]. Its incorporation into nanocomposites and antimicrobial films has enhanced food quality, extended shelf life, and addressed critical challenges in food safety and sustainability. Furthermore, PEG-based hydrogels are being explored for nutrient delivery and antimicrobial applications in food packaging, showcasing their potential to innovate this sector [36–38].
Despite its widespread applications, conventional PEG synthesis often results in heterogeneous mixtures of polymers with varying molecular weights. This variability poses challenges in achieving consistency in production, regulatory compliance, and precise functionality. Advances in synthetic methods, such as the development of PEG-based copolymers, have addressed many of these limitations and paved the way for more reliable applications in both medical and food industries. [39–42].
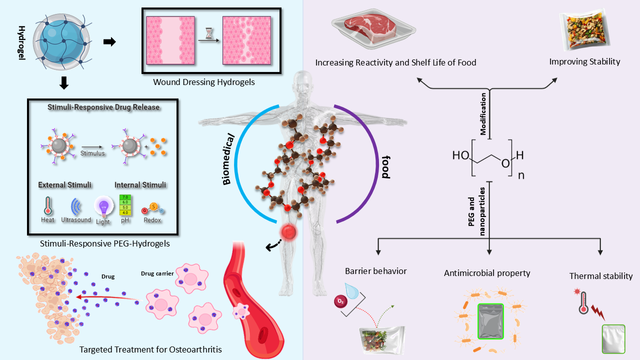
In this review article, we explore the diverse applications of polyethylene glycol (PEG)-based materials, spanning from biomedical innovations to advancements in the food industry, with a focus on their pivotal roles in drug delivery systems, biomedical technologies, and food packaging solutions. Over the past decade, PEG has emerged as a cornerstone material, enabling groundbreaking advancements in areas such as stimuli-responsive hydrogels, targeted drug delivery, and antimicrobial packaging. As illustrated in Scheme 1, we highlight the versatility of PEG-based materials, showcasing their transformative applications in biomedical fields—such as stimuli-responsive drug delivery systems and wound healing hydrogels—as well as their innovative roles in the food industry, including antimicrobial packaging and shelf-life enhancement. This review addresses the unique physicochemical properties of PEG—such as its biocompatibility, water solubility, and tunable molecular structure—that make it an ideal candidate for diverse applications in biomedicine and the food industry. We explore the transformative roles of PEG in drug delivery systems, wound healing, tissue engineering, and food packaging, highlighting its ability to enhance therapeutic outcomes, improve food quality, and extend shelf life. Additionally, we discuss the challenges associated with PEG, including limited biodegradability, potential adverse health effects, and difficulties in fine-tuning its properties for specific applications. By synthesizing recent advancements and addressing these limitations, this article aims to provide a comprehensive roadmap for leveraging PEG’s unique properties to drive innovations that improve human health and sustainability. As PEG continues to evolve, its potential to revolutionize these critical fields remains vast and promising.
Physicochemical and biological properties of PEG
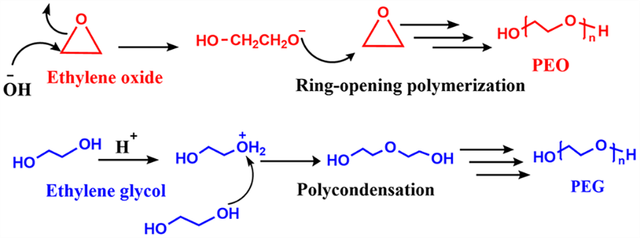
PEG is a polyether made by polycondensation polymerization (using ethylene glycol) and ring-opening polymerization (ROP) using ethylene oxide. PEG, polyoxyethylene (POE), and polyethylene oxide (PEO) are the three names for this polyether. ROP is used to create PEO, and polycondensation polymerization is used to create PEG. Because of the way it was prepared, the terminals of this polyether have hydroxyl (OH) groups at the ends, therefore it can be called a polyol, which is a macromonomer. At any molecular weight, this dicarbonyl ether (C₂-ether) dissolves readily in water. H−(O−CH2−CH2)n−OH is the chemical formula for the polymer, where n is the degree of polymerization, which can vary [43,44]. PEG, POE, and PEO are oligomers or polymers. Although the three terms are chemically identical, PEG is traditionally used in biomedical applications, while PEO is more commonly used in industrial polymer chemistry. Since different applications require different polymer chain lengths, PEG refers to oligomers and polymers with molecular weights of less than 20,000 g/mol, PEO refers to polymers with molecular weights greater than 20,000 g/mol, and POE refers to polymers of any molecular weight [45] and PEG is commercially accessible in a wide range of molecular weights, from 200 g/mol to ten million g/mol [46]. Low molecular weight PEG (less than 1000 g/mol) is a colorless, odorless liquid that is highly soluble in water and typically flows easily at ambient temperatures. These properties make PEG very useful in processes such as drug delivery, surface modification, and the production of aqueous formulations for biological and pharmaceutical materials. In contrast, as the molecular weight of PEG increases (more than 1000 g/mol), the polymer becomes solid or semi-solid and is typically observed in waxy or gel forms [47,48]. These phase changes occur due to the increase in the number of ethylene glycol repeating units, which affects the polymer’s elastic properties and melting temperature. Additionally, PEG has remarkable mechanical properties that, when combined with other materials, can improve the elastic properties, strength, and ductility of the final product [49]. These properties have made PEG popular in the design and synthesis of network polymers and gel polymers for drug delivery and biological materials [50].
Regarding the chemical properties of PEG, due to the presence of oxyethylene units in each of its repeating units and the presence of hydroxyl groups at both ends of its molecular chain, it is able to form hydrogen bonds with other molecules [51]. These properties make PEG highly soluble in aqueous media and able to interact with various substances such as acids, bases, and organic compounds [52,53]. The hydroxyl groups present in PEG play a crucial role in facilitating esterification and etherification reactions. This characteristic renders PEG highly valuable for the synthesis of organic ethers, esters, and even block copolymers [54–56]. PEG has also attracted attention as a biopolymer due to its very low toxicity—or indeed its non-toxic nature—and its exceptional environmental biocompatibility, which distinguishes it from many other synthetic polymers. [57,58]. These characteristics have made PEG highly valuable in applications such as drug delivery and the surface modification of proteins and biological materials [59,60]. PEG can interact with amino and carboxyl groups on the surfaces of proteins or nanoparticles, thereby enhancing the physical and chemical properties of these systems [61]. Specifically, in drug delivery, PEG is widely used as a coating to prevent drug degradation, improve solubility, and reduce toxicity [62]. One of the important chemical properties of PEG is its structural versatility. For instance, PEG can undergo chemical reactions with various functional groups, such as silanes, amines, esters, and carboxylates, to impart tailored properties, including reduced adhesion, enhanced stability in chemical environments, or improved biocompatibility [63–65]. These modifications are particularly significant for advancing applications in pharmaceuticals, biotechnology, and the food industry [66]. Figure 1 illustrates the synthesis mechanisms of PEG and PEO.
*PEG: Polyethylene glycol, PEO: Polyethylene oxide
PEG’s Hydrophilic Nature and Impact on Viscosity
PEG, owing to its hydrophilic nature, plays a critical role in enhancing the solubility and flexibility of materials. This hydrophilic property significantly reduces the viscosity of polymer solutions, thereby facilitating the processing of food packaging films. By lowering viscosity, PEG enhances both the flexibility and elongation at break of the films, making them more adaptable and easier to handle during production. Furthermore, PEG’s water absorption capacity contributes to enhancing the water vapor resistance of the films, which in turn boosts their durability and overall performance in food packaging applications [21,22].
Biomedical Applications of PEG
PEG-Based Hydrogels
Hydrogels, known for their high absorbability and excellent biocompatibility, have gained widespread use in medical and biological treatments [67]. In recent years, the development of novel hydrogels with unique properties, such as controlled drug release and self-healing abilities, has attracted considerable attention from researcher [68,69]. A key strategy in this field involves the use of polymers like PEG, which is extensively employed in hydrogel design due to its high biocompatibility and structural versatility [70]. Furthermore, the combination of PEG with other materials, such as chitosan (CS) and polyvinyl alcohol (PVA), has proven particularly valuable for creating hydrogels with enhanced properties, especially for applications like burn treatment and the prevention of hypertrophic scars [71,72].
Innovative chemical reactions, such as click chemistry, have been utilized to construct three-dimensional (3D) hydrogel networks. These methods effectively impart desirable characteristics, including high hydrophilicity and increased nitrogen content, while also introducing additional functionalities, such as flammability, into eco-friendly materials [73]. In this section of the review, highlights recent advancements in the design of PEG-based hydrogels. These hydrogels, not only exhibit exceptional hydrophilicity but also demonstrate controlled drug release and degradability in reducing environments. These properties could have a significant impact on medical and pharmaceutical applications, Such as drug delivery Systems for wound healing. Additionally, recent studies suggest that incorporating specific compounds, such as triazole heteroaromatic rings and ethylene oxide units, into hydrogel structures can enhance both physical and biological properties. This integration may also introduce specific functionalities, such as antibacterial activity, into pharmaceutical systems, paving the way for innovative material designs tailored to specialized applications [74].
Polymeric Drug Delivery for Wound Healing
In 2022, researchers conducted a study on the development of a Poly (Lactic Acid) (PLA)-PEG-based drug delivery system for wound healing modulation in glaucoma eye surgery [75]. The aim of the study was to utilize cyclosporine A (CsA) and everolimus in PLA-PEG implants to modulate the wound healing process following glaucoma surgery. The PLA-PEG implants were able to absorb CsA and everolimus and release them gradually without any toxic effects. In animal experiments, these implants improved the outcomes of glaucoma surgery and reduced excessive scarring. The drugs were continuously released for 7 days (CsA) and 13 days (everolimus), respectively. In the surgical procedure, a small incision was made at the limbus of the eye, and drug-loaded implants were placed in the surgical area. After surgery, the wounds were closed with sutures, and the animals received drug treatment. This approach demonstrates that PLA-PEG implants saturated with CsA or everolimus can enhance the outcomes of glaucoma surgery and reduce surgery-induced scarring (figure 2). In another study conducted in 2023, PLA-PEG was utilized as a drug delivery system based on polymeric micelles [76]. Polymeric micelles are formed from amphiphilic block copolymers, with the PEG component playing a crucial role in enhancing drug solubility and high biocompatibility. These properties help the micelles evade recognition by the immune system and prevent their rapid elimination. Additionally, these features contribute to increased stability of the micelles and the controlled release of drugs at the targeted site. In this study, PLA-PEG was used to load drugs like nifedipine, improving the solubility and bioavailability of the drugs. Figure 2 illustrates the block copolymer structure and the formation of micelles. In this structure, the hydrophobic part of the micelle encapsulates hydrophobic drugs, while the hydrophilic part prevents direct contact of the micelle with the aqueous environment. This design helps the micelles become more resistant and stable against immune system recognition. PEG was also used in the treatment of burn wounds, focusing on accelerating epithelialization, preventing infection, and reducing scar formation [77]. In this study, CS, PVA, and PEG were combined to produce hydrogels with controlled vancomycin release. These hydrogels were crosslinked using glutaraldehyde and then freeze-dried. The results showed that the hydrogels had a porous structure, and the incorporation of PEG reduced the pore size. This helped with water absorption and provided a suitable moist environment to accelerate epithelialization. Additionally, the hydrogels significantly slowed the release of vancomycin while maintaining its antibacterial efficacy against Staphylococcus aureus.
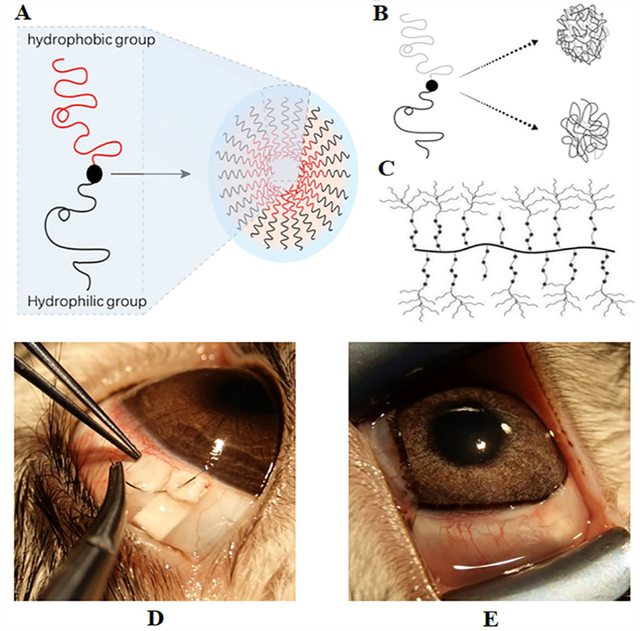
*PLA: Poly (Lactic Acid), PEG: Poly ethylene glycol
Redox-Responsive Polymer Networks
Nanogels are nano-sized, three-dimensional cross-linked polymeric structures that are widely used for drug delivery, diagnostics, and biomedical applications [78]. Due to their flexible nature and ease of fabrication with important biomolecules, nanogels have demonstrated their potential in the successful delivery of drugs to disease sites following modification with target cell-recognizing chemical groups. PEG plays a crucial role in nanogel formulations due to its hydrophilic properties, biocompatibility, and ability to improve the stability of nanogels. PEG helps achieve controlled drug release by enhancing the swelling behavior of the nanogels and increasing their surface area [79]. Additionally, PEG can modulate the properties of nanogels, such as size, surface charge, and degradability, depending on the intended application. Since PEG has flexible characteristics, nanogels can respond to various stimuli such as pH, temperature, light, magnetism, enzymes, and redox conditions, allowing for targeted and controlled drug release at the site of action [80]. Figure 3a illustrates how nanogels respond to various stimuli (such as pH, temperature, or redox conditions) and facilitate targeted drug release. This figure likely demonstrates the drug release process from nanogels in response to specific conditions present at the disease site.
Redox-responsive nanogels are composed of chemically active crosslinkers, including disulfide, ditellurium, and diselenide bonds [81]. These bonds break in the presence of reducing agents like reduced glutathione (GSH), dithiothreitol (DTT), or other similar compounds, enabling the controlled release of drugs from the nanogels. Specifically, disulfide bonds between cysteine groups in proteins are converted to thiol groups through redox reactions [82]. Similar bonds are present in polymeric systems, where they are cleaved upon internalization by cells, leading to drug release. Therefore, nanogels are typically composed of polymers that are linked to disulfide or similar bonds. These bonds break in response to redox conditions in the intracellular environment, releasing the drug in a controlled manner at the target site. These features enable nanogels to have significant potential in targeted therapies, particularly in cancer chemotherapy [83].
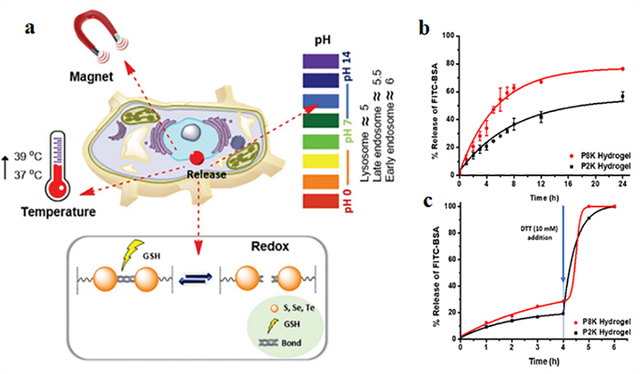
*PEG8K-PDS: PEGMw:8000- Pyridyl Disulfide, DTT: dithiothreitol, FITC-BSA: fluorescein isothiocyanate-labeled bovine serum albumin, GSH: reduced glutathione.
In 2022, Kilic Boz and colleagues designed redox-responsive hydrogels using PEG conjugated with pyridyl and thiol disulfide groups [84]. The primary aim of this research was to improve the hydrogels’ properties, including better rheology, self-healing, controlled degradability, and drug release. PEG enhanced the mechanical and rheological properties of the hydrogels and improved their self-healing capabilities. These hydrogels degraded in reducing environments such as DTT and GSH, making them useful for drug delivery and medical treatments. In this study, the release of protein from PEG-based hydrogels was faster with an increase in the molecular weight of PEG (Figure 3b, c). Additionally, when a reducing agent like DTT was added to the hydrogel, its structure degraded, and the protein was released rapidly and in a controlled manner, indicating the hydrogel’s responsiveness to specific conditions. PEG also enabled the controlled release of drugs and proteins from the hydrogels. These properties make the hydrogels suitable for medical applications such as drug delivery, tissue engineering, and therapeutic treatments. Specifically, the timed drug release allows for more targeted and effective treatments. Furthermore, these hydrogels can be used in long-term treatments without the need for repeated interventions.
Wound Dressing Hydrogels
One of the most promising approaches for the synthesizing antibacterial hydrogels is click chemistry, a method that not only facilitates the formation of three-dimensional structures but also incorporates nitrogen heteroatoms and triazole rings into the hydrogel network. These modifications impart unique properties such as antibacterial activity and controlled flammability, to the system. In 2024, researchers developed a novel PEG-based hydrogel with high nitrogen content, incorporating heteroaromatic groups and repeating ethylene oxide units (-OCH2CH2-). During this synthesis, triazole rings were introduced into the hydrogel structure, enabling specific functionalities such as sustained antimicrobial activity at wound sites [85]. Table 1 summarizes the water absorption properties of various hydrogels [77, 84–92]. The exceptional water absorption capacity of PEG-based hydrogels ensures effective moisture at the wound site, creating a moist environment conducive to accelerated wound healing.
This property is critical for reducing tissue dryness, preventing further damage, and promoting the biological processes involved in tissue repair. In a related study from 2023, click chemistry was employed as an innovative strategy for designing advanced therapeutic systems [93]. This hydrogel system combines adipose-derived stem cell (ADSC)-derived nanovesicles (ANVs) with an aggregation-induced emission (AIE) photosensitizer, targeting the treatment of deep burns and bacterial infections. Click chemistry enabled the precise and efficient integration of the nanovesicles into the hydrogel matrix. PEG played a pivotal role in this system, enhancing biocompatibility, improving stability under physiological conditions, and facilitating the controlled and sustained release of therapeutic agents. Importantly, PEG allowed for pH-responsive drug release in the acidic wound environment, further accelerating the healing process. Figure 4 illustrates the construction of the THB@ANVs-encapsulated hydrogel and its mechanism for deep scald wound repair, providing a visual representation of the wound recovery process.
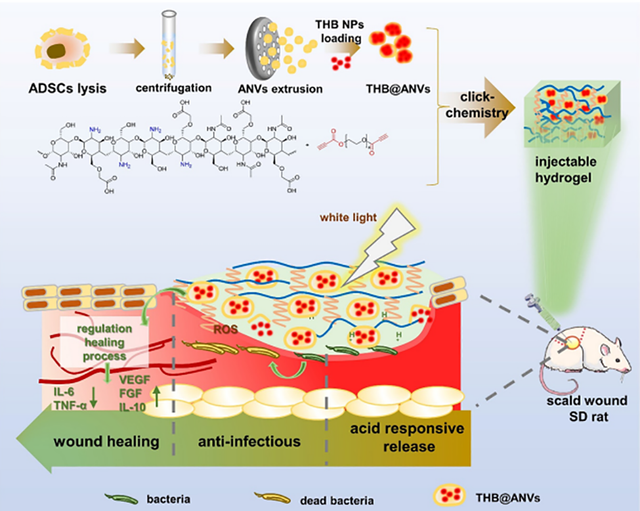
*THB: 4-(2-(5-(4- (diphenylamino)phenyl) thiophen–2-yl) vinyl)–1-(2-hydroxyethyl) pyridin–1-ium bromide, ANVs: (ADSC)-derived nanovesicles.
Entry | PEG-based hydrogel | Water uptake (%) | Ref. |
1 | 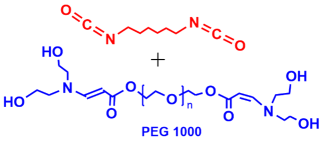 | 41 | 86 |
2 | 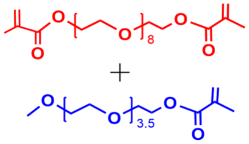 | 340 | 87 |
3 | 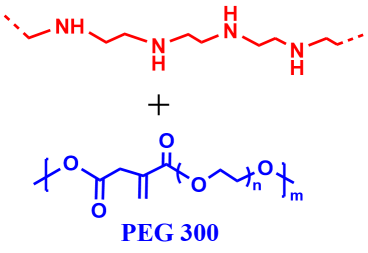 | 149 | 88 |
4 | 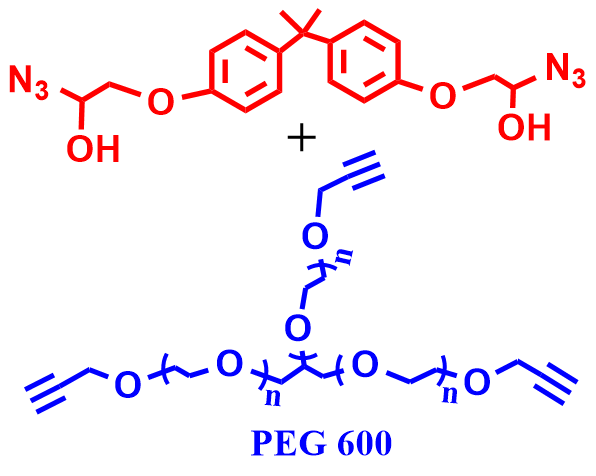 | 98 | 89 |
Entry | PEG-based hydrogel | Water uptake (%) | Ref. |
5 | 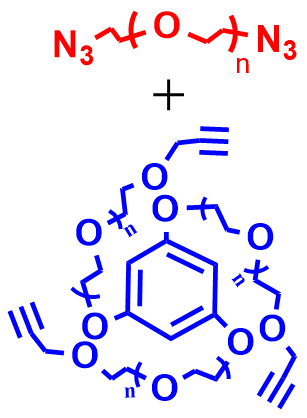 | 173 | 85 |
6 | 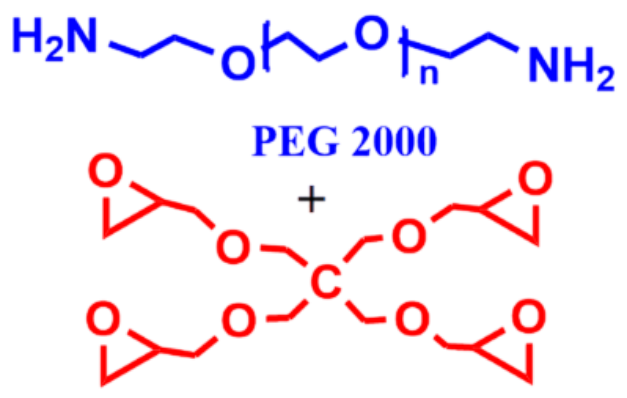 | 649 | 90 |
7 | 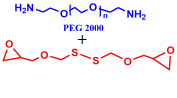 | 765 | 91 |
8 | 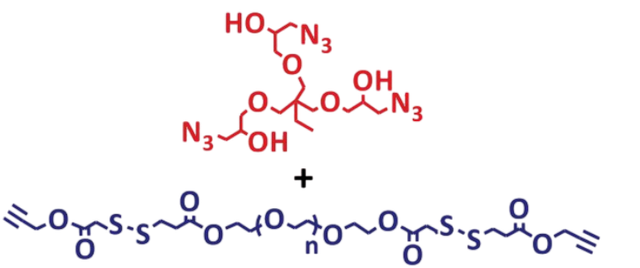 | 526 | 92 |
Entry | PEG-based hydrogel | Water uptake (%) | Ref. |
9 | 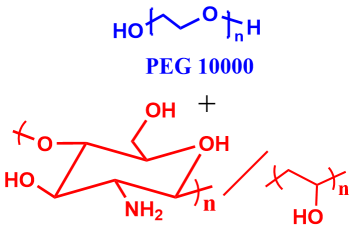 | 930 | 77 |
10 | 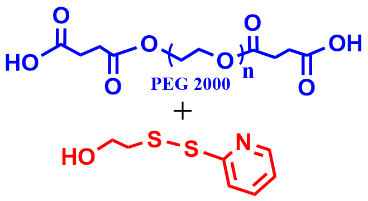 | 2200 | 84 |
Drug delivery systems
This section of the review article highlights the application and significance of the PEGylation technique in biopharmaceuticals. PEGylation, the process of attaching of PEG to drugs and biological molecules, particularly biologics such as proteins and antibodies, has emerged as a powerful strategy to enhance drug properties [94]. This technique improves therapeutic efficacy, minimizes side effects, and optimizes targeted drug delivery systems by extending the half-life of drugs, reducing immunogenicity, and enhancing their stability [95,96]. Over the past decade, PEGylation has gained considerable attention in drug delivery systems, especially for the treatment of resistant cancers [97]. For instance, PEG and Poly (lactic-co-glycolic acid) (PLGA)-based drug delivery systems, leverage the hydrophilic nature of PEG to enable controlled drug release and improve therapeutic outcomes, particularly in challenging cancers such as triple-negative breast cancer (TNBC) [98]. Despite its numerous advantages, PEGylation is not without challenges. Issues such as the accumulation of PEGylated drugs in the liver and the potential reduction in biological activity of certain drugs post-PEGylation have been reported [99]. As a result, optimizing the molecular weight of PEG and developing innovative approaches in this field are critical to overcoming these limitations. The following sections will explore and review key applications and impacts of PEG and PEGylation in drug delivery systems, providing a comprehensive understanding of their role in advancing biopharmaceutical therapies.
Targeted Treatment for Osteoarthritis
In 2024, researchers designed and developed a targeted drug delivery system for the treatment of osteoarthritis, aiming to specifically affect chondrocyte cells and restore mitochondrial function within these cells [100]. This system, utilizing nanotechnology, combines various materials such as liposomes, microsphere hydrogels, and specific peptides to simultaneously target chondrocyte cells and improve mitochondrial function. In this system, liposomes are used as drug carriers for Urolithin A (UA), a natural compound known for its effects on mitophagy (the autophagic elimination of dysfunctional mitochondria). UA helps restore mitochondrial function, which plays a crucial role in diseases such as osteoarthritis. Peptide WY specifically binds to the liposomes, preparing them for targeting chondrocyte cells. Peptide WY interacts with glycosaminoglycans (GAGs) present in cartilage, ensuring that the drug (UA) is selectively absorbed by chondrocyte cells and prevents unwanted distribution to other tissues. Thus, peptide WY plays a selective targeting role. PEG is added to the liposomes to prevent aggregation and enhance structural stability [101]. PEG also prolongs the drug’s retention in the body by preventing rapid recognition and clearance of the liposomes by the immune system, allowing the drug to stay in the body longer and provide better therapeutic effects. In this study, microsphere hydrogels were made from hyaluronic acid methacrylate (HAMA), which have a porous structure allowing for slow drug release in the joint environment. The combination of PEG with liposomes also helps deliver the drug more specifically and with a longer retention time to the chondrocyte cells.
Figure 5 illustrates the overall process of designing and constructing the HM@WY-Lip/UA system. The figure clearly shows the following steps: first, liposomes are made using peptide WY to target chondrocyte cells and Urolithin A (UA) for mitophagy. Then, hydrogels are created using hyaluronic acid methacrylate (HAMA), which are porous and suitable for intra-articular injection. Next, the liposomes loaded with Urolithin A and WY are incorporated into the hydrogels, allowing the drug to be released locally and slowly. Finally, with the help of peptide WY, the system targets chondrocyte cells, specifically activating in these regions to improve mitochondrial function using Urolithin A. Ultimately, the goal of this system is to enhance drug retention in the joint, precisely target chondrocyte cells, and effectively treat osteoarthritis by improving mitochondrial function and reducing oxidative stress.
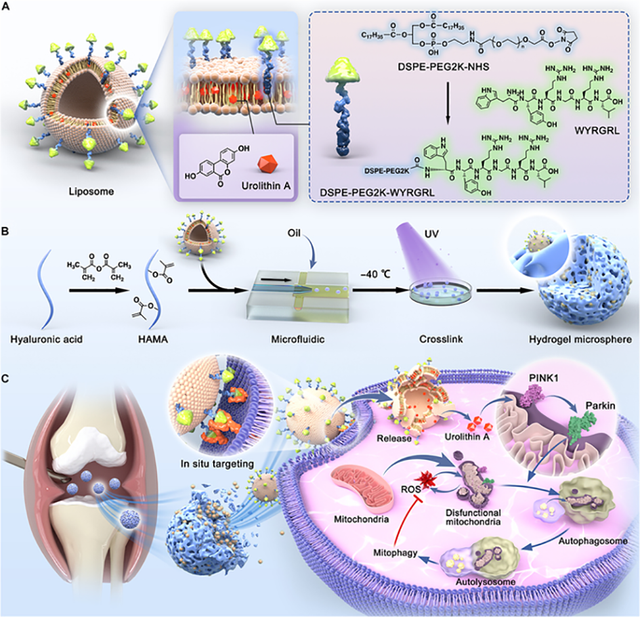
*PEG: Polyethylene glycol
PEGylation in Therapeutic Drug Systems
In recent years, researchers have extensively explored the effects of PEGylation in drug delivery yielding promising results that demonstrate its potential to significantly enhance the efficiency of targeted drug delivery systems [102]. PEGylation involves the attachment of PEG to drugs and biological molecules, aiming to improve their properties by extending their half-life, reducing clearance from the body, and minimizing immunogenicity. This technique allows drugs particularly biologics such as proteins, peptides, and antibodies to remain in the body for longer periods, thereby
This technique allows drugs particularly biologics such as proteins, peptides, and antibodies to remain in the body for longer periods, thereby substantially increasing therapeutic efficacy. PEG, a nontoxic and nonantigenic polymer, possesses hydrophilic properties that enhance the water solubility of PEGylated drugs. This characteristic is especially beneficial for high
molecular weight drugs, as it reduces renal excretion and prolongs their presence in the bloodstream [103]. Additional advantages of PEGylation include reduced immunogenicity and improved drug stability, which decrease the need for frequent drug administration. For PEGylation of drugs and proteins, PEG with active end groups capable of reacting with specific chemical groups such as amines, thiols, and carboxylates is commonly employed. PEGylation has found widespread applications in various medical fields, including the treatment of hepatitis C, acromegaly, and the production of monoclonal antibodies for autoimmune diseases and cancer [104–106]. PEG is also used as a carrier for targeted drug delivery in cancer therapy, reducing systemic toxicity and enhancing the precision of drug delivery to tumor sites. Despite its numerous benefits, PEGylation is not without limitations. For instance, high molecular weight PEGylated drugs may temporarily accumulate in the liver, potentially leading to macromolecular syndrome [107]. Additionally, some drugs may experience reduced biological activity after PEGylation. Therefore, further research into optimizing the molecular weight of PEG and developing innovative approaches, such as degradable PEGylation, is essential to address these challenges. [108]. Overall, PEGylation is recognized as a powerful strategy in biopharmaceuticals, capable of improving the therapeutic effects of many drugs while mitigating safety concerns and rapid degradation. This technique is currently employed in the production of numerous advanced drugs and plays a pivotal role in enhancing targeted and novel therapies for various diseases, including cancer and autoimmune disorders [108,109]. Table 2 provides a list of FDA-approved PEGylated drugs, covering a broad spectrum of medical applications [110]. For detailed information on each of these drugs, reliable sources such as the official FDA website can be consulted.
Entry | Trade Name | Company | PEGylated Entity | Indications | Average MW of PEGs | Approved | Ref. |
1 | lfabrio (pegunigalsidase alfa-iwxj) | Chiesi Global Rare Diseases/Protalx | Recombinant human GLA enzyme | Fabry disease | ~2 kDa | 2023 | FDA |
2 | Izervay (avacincaptad pegol) | Iveric Bio | Ribonucleic acid aptamer | Geographic atrophy | 43 kDa | 2023 | FDA |
3 | Syfovre (pegcetacoplan injection) | Apellis | Pentadecapeptide | Geographic atrophy | 40 kDa | 2023 | FDA |
4 | Rolvedon (eflapegrastim-xnst) | Spectrum Pharmaceuticals | G-CSF | Febrile neutropenia | 3.4 kDa | 2022 | FDA |
5 | Stimufend (pegfilgrastim-fpgk) | Fresenius Kabi | G-CSF | Neutropenia | 20 kDa | 2022 | FDA |
6 | Fylnetra (pegfilgrastim-pbbk) | Amneal Pharmaceuticals LLC | G-CSF | Neutropenia | 20 kDa | 2022 | FDA |
7 | Besremi (ropeginterferon alfa–2b) | PharmaEssentia Corp | Interferon | Polycythemia vera | 40 kDa | 2021 | FDA |
8 | Skytrofa (lonapegsomatropin) | Ascendis | Human growth hormone | Growth hormone deficiency | 4 × 10 kDa | 2021 | FDA |
9 | Empaveli (pegcetacoplan) | Apellis | Penta decapeptide | Paroxysmal nocturnal hemoglobinuria | 40 kDa | 2021 | FDA |
Entry | Trade Name | Company | PEGylated Entity | Indications | Average MW of PEGs | Approved | Ref. |
10 | Spikevax | Moderna | Lipid nanoparticles | Prevention of COVID–19 | 2 kDa | 2020 | FDA |
11 | Comirnaty | BioNTech/Pfizer | Lipid nanoparticles | Prevention of COVID–19 | 2 kDa | 2020 | FDA |
12 | Nyvepria (pegfilgrastim-apgf) | Pfizer Inc. | G-CSF | Neutropenia associated with chemotherapy | 20 kDa | 2020 | FDA |
13 | Esperoct (turoctocog alfa pegol) | Novo Nordisk | Recombinant antihemophilic factor | Hemophilia A | 40 kDa | 2019 | FDA |
14 | Ziextenzo (pegfilgrastim-bmez) | Sandoz | G-CSF | Infection during chemotherapy | 20 kDa | 2019 | FDA |
15 | Onpattro (patisiran) | Alnylam Pharmaceuticals | Lipid nanoparticles | Polyneuropathy of hereditary transthyretin-mediated amyloidosis | 2 kDa | 2018 | FDA |
16 | Movantik (naloxegol) | AstraZeneca | Naloxone | Constipation | 339 Da | 2014 | FDA |
Stimuli-Responsive PEG-Hydrogels for Targeted Drug Delivery
The metabolism of PEG-based hydrogels in the body is a critical factor in their performance for drug delivery. PEG is naturally processed by the body, with its degradation rate being closely tied to its molecular weight. Hydrogels with lower molecular weights tend to break down more quickly and are excreted faster. Research has demonstrated that PEG hydrogels are generally excreted within 10 days after implantation, which makes them a safe material for drug delivery applications [102].
The synthesis of PEG-based hydrogels involves various techniques, including polymerization methods and chemical cross-linking. These approaches enable the encapsulation of therapeutic agents within the hydrogel network, with drug release triggered under specific conditions. Depending on the type of drug and the desired release profile, PEG-based hydrogels can be engineered to respond to external stimuli, such as light or magnetic fields, as well as internal physiological cues, including pH changes, temperature fluctuations, or enzyme activity [111, 112].
As illustrated in Figure 6, recent advancements in PEG-based drug delivery systems can be categorized into two main groups: systems responsive to external stimuli (e.g., light, magnetic fields) and those responsive to internal physiological conditions (e.g., pH, temperature, oxygen levels, or specific enzymes) [112]. These innovations have significantly expanded the potential of PEG hydrogels in targeted and controlled drug delivery.
Despite the remarkable progress in PEG hydrogel-based drug delivery systems, particularly for cancer therapy, several challenges remain. Key areas for future research include enhancing the clinical efficiency of these systems, improving the precision of drug release, and addressing issues related to the degradation and elimination of hydrogel materials from the body. Overcoming these challenges will be critical for advancing the clinical translation of PEG-based hydrogels and maximizing their therapeutic potential.
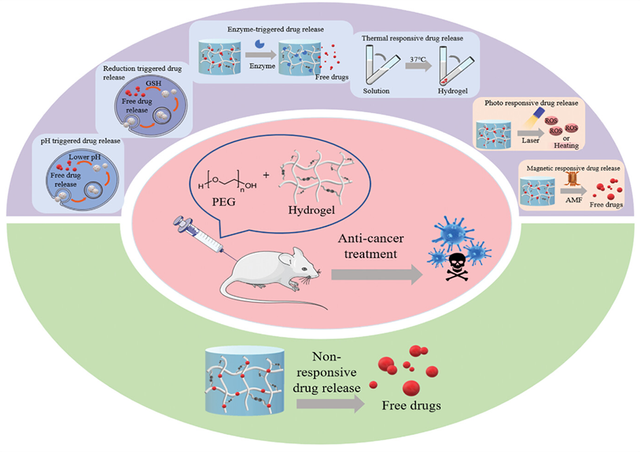
*PEG: Polyethylene glycol
*PEG: Polyethylene Glycol, PLGA: Poly (lactic-co-glycolic acid), MRI: Magnetic Resonance Imaging
Thermosensitive Hydrogel Monitoring
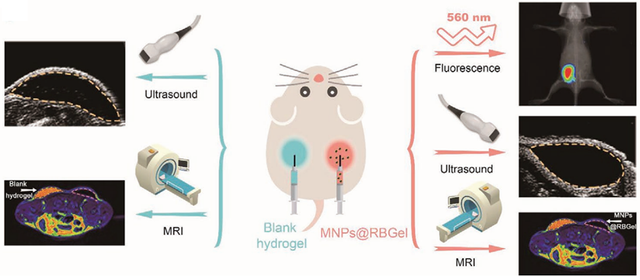
Chen and et al, in a study, synthesized a thermosensitive PLGA-PEG-PLGA hydrogel using PLGA and PEG to create an injectable and thermally responsive hydrogel system [114]. To achieve this, PLGA polymer was selected as the core structure of the polymer, and PEG was attached to both ends of the PLGA chains to form a thermosensitive structure that gels at body temperature (37°C). This hydrogel was covalently conjugated with the fluorescent probe RB (Rhodamine B) to enable fluorescence tracking of degradation processes in the body. Additionally, cobalt ferrite nanoparticles (CoFe2O4) were incorporated into the hydrogel to improve the contrast of Magnetic Resonance Imaging (MRI) and monitor the hydrogel degradation process through multiple non-invasive imaging techniques. The results showed that this hydrogel exhibited excellent thermosensitive properties, gelling at body temperature, and being capable of continuous drug release over time. The addition of PEG to the hydrogel, due to its hydrophilic nature, helped maintain water within the hydrogel structure and enhanced its biocompatibility and controlled degradation properties. Non-invasive imaging techniques such as ultrasound, fluorescence, and MRI enabled precise and continuous tracking of the hydrogel’s degradation process over time. Figure 7 illustrates the various imaging processes and analyses of this hydrogel, where fluorescence and MRI changes were employed to investigate the degradation process and distribution of the materials.
Applications in Food Industry
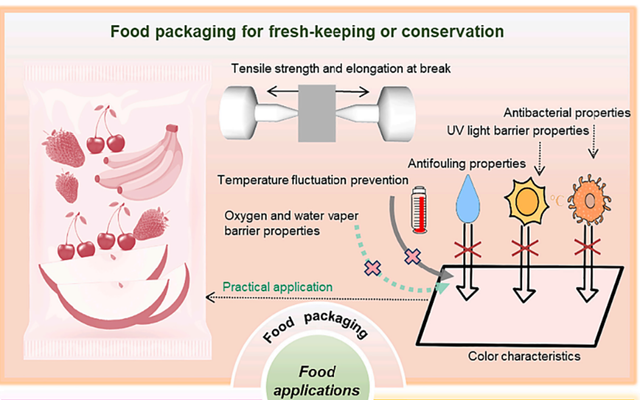
In today’s world, the food packaging industry faces numerous challenges, including extending the shelf life of food products and minimizing waste [115]. An innovative approach to addressing these issues is the development of advanced materials for packaging films that preserve the quality and freshness of food. In this regard, PEG and its derivatives have attracted much attention as multifunctional compounds [116,117]. PEG’s unique attributes, such as its wetting capability, high solubility, and antibacterial properties, make it a valuable component in food packaging, contributing to improved safety and prolonged shelf life. Recent studies have focused on the impact of PEG on the physical and chemical characteristics of food packaging films, with findings that could transform the industry [118–120]. These investigations highlight PEG’s ability to enhance the mechanical strength, water vapor resistance, and antibacterial efficacy of packaging films, opening up new possibilities for its application in the food sector.
Enhancing Food Quality and Shelf Life
The application of PEG and its derivatives as multifunctional compounds is widespread in the food industry, where they are integrated into a diverse range of products. One of PEG’s primary roles is as a humectant, effectively preventing the drying out of baked goods such as pastries and cakes [117]. Due to its high water absorption capacity, PEG retains moisture, significantly extending the shelf life of these products [121]. Additionally, PEG serves as a versatile solvent, facilitating the blending of various components in both aqueous and non-aqueous systems, making it an ideal choice for beverages and desserts [122]. In chocolate and emulsion-based products, PEG prevents ingredient separation and adhesion, streamlining the production process and enhancing product consistency [123].
Furthermore, emerging studies have demonstrated that PEG, in combination with polysaccharide-based materials, exhibits exceptional encapsulation properties, offering significant potential for various applications. These materials can contribute to the development of advanced food packaging, enhance smart diagnostics and signaling in food products, foster innovation in personalized nutrition, and improve the bioavailability of specialized food ingredients in humans [124] (Figure 8).. Overall, PEG has proven to be a valuable asset in the food industry, particularly for improving textural properties and overall product quality. However, strict adherence to safety standards and careful monitoring of dosage are essential to ensure its safe use. When applied appropriately, PEG can significantly enhance both the quality and shelf life of food products.
*PEG: Polyethylene glycol
*PVA: Polyvinyl alcohol, PEG: Polyethylene glycol, AZGP: Ag–ZnO–reduced graphene oxide–Polyethylene glycol
PEG-Based Nanocomposites for Packaging
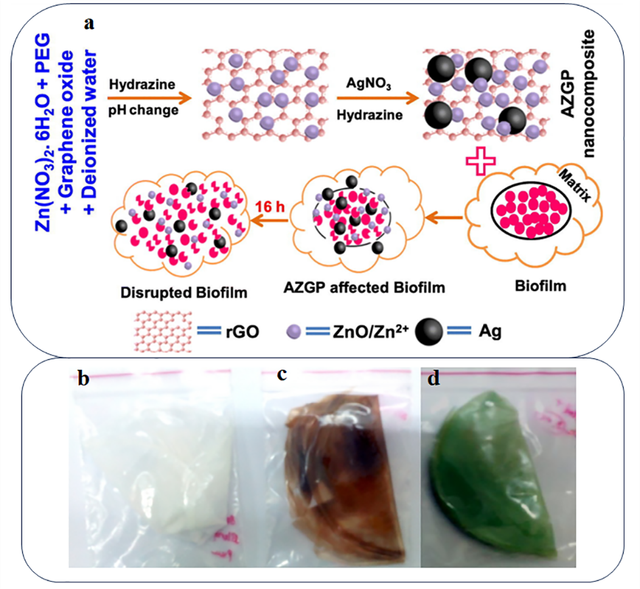
In recent years, numerous studies have been conducted on the application of various nanoparticles in the development of PEG-based nanocomposites for the food industry. For instance, one study investigated biodegradable nanocomposite films composed of PLA, PEG, and nano glass flakes (NGF) for food packaging applications. This combination demonstrated enhanced properties, including high transparency, improved hydrophobicity, superior oxygen barrier performance, and increased thermal stability in the resulting packaging films [125]. In another example, atmospheric pressure plasma polymerization was used to develop antibacterial coatings on polymer substrates, incorporating PEG and ZnO nanoparticles, which were safe and effective for food packaging [126]. Researchers also explored the use of silver nanoparticles combined with biopolymers like PVA and PEG to produce antibacterial films [127]. The silver nanoparticles were synthesized via a green synthesis approach using Capparis zeylanica leaf extract and integrated into PVA/PEG composite films. The addition of silver nanoparticles improved the films’ antibacterial efficacy, moisture absorption, and thermal stability. PEG acted as a plasticizer and softener, enhancing flexibility and mechanical properties. These composite films demonstrated the potential to extend the shelf life of food by reducing spoilage. The antibacterial activity was confirmed using the disk diffusion method against pathogens such as E. coli, P. aeruginosa, and S. aureus, with the largest inhibition zone observed for P. aeruginosa. Additionally, the color changes in the films with silver and copper nanoparticles (Figures 9b-d) demonstrated improved food preservation and extended shelf life. These findings highlight the potential of PEG-based nanocomposites for sustainable and antibacterial food packaging solutions. In another study, Ag–ZnO–reduced graphene oxide (rGO)–PEG (AZGP) nanocomposites were synthesized using a room temperature dissolution process [128]. In this process, the silver nitrate content was varied, while the amounts of graphene oxide and PEG remained constant. PEG was used to improve the stability of the nanocomposites and enhance their mechanical and chemical properties. It also facilitated the formation of chemical bonds between rGO and the nanoparticles, which enhanced the antibacterial activity of the nanocomposites. The results showed that the AZGP nanocomposite demonstrated high anti-biofilm activity (around 95%) against Staphylococcus aureus and Pseudomonas aeruginosa bacteria and was effective in eradicating preformed biofilms. Additionally, AZGP-based agar films were fabricated for food packaging applications, exhibiting high tensile strength and maintaining excellent antibacterial activity even after 90 days. This simple and cost-effective synthesis strategy could be useful for the development of other nanocomposites with applications in food packaging. In the Affected biofilm process, the nanocomposites inhibit biofilm growth and prevent the formation of new biofilms. After 16 hours, in the disrupted biofilm process, the nanocomposites degrade preformed biofilms and eliminate them by releasing metal ions (Figure 9a).
Antimicrobial PEG Films for Food Packaging
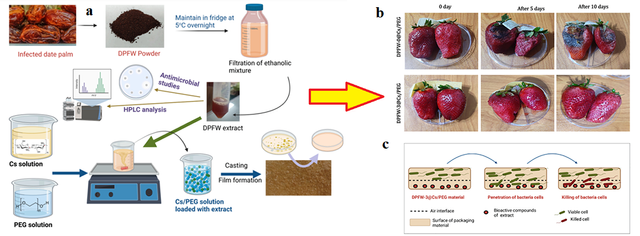
Zidan and et al, developed antimicrobial active packaging films using chitosan (Cs) and polyethylene glycol (PEG) [130]. Chitosan was selected as the base material due to its antimicrobial and biocompatible properties, while PEG was added to enhance the flexibility and tensile strength of the films. DPFW extract (date palm waste), containing bioactive compounds such as polyphenols and tannins, was incorporated into the films to enhance their antimicrobial properties. The films were prepared in two variations: one without DPFW extract (DPFW–0@Cs/PEG) and another with a higher amount of extract (DPFW–3@Cs/PEG). To prepare the films, chitosan and polyethylene glycol were first dissolved in an aqueous solution. Then, DPFW extract was added to ensure a uniform mixture. After adding glycerol as a plasticizer and glutaraldehyde as a crosslinking agent, the solutions were stirred at 75°C. The mixtures were then poured into petri dishes and dried at room temperature for 72 hours. This process is detailed in Figure 10a. The resulting films exhibited strong antimicrobial properties and were capable of continuously releasing antibacterial compounds, preventing microbial growth and thereby extending the shelf life of fruits. Strawberries were packaged using these films and stored at 25°C. The results showed that strawberries packaged with DPFW–0@Cs/PEG experienced spoilage, black spots, and mold growth after 5 days, while strawberries packaged with DPFW–3@Cs/PEG remained fresh. These results are illustrated in Figure 10b,c. The DPFW–3@Cs/PEG films exhibited strong antibacterial properties, preventing microbial growth and increasing the shelf life of strawberries. also in 2024, a study investigated the impact of PEG on O-CMC–PEG films for food packaging applications [131]. Researchers synthesized these films using a condensation reaction between PEG and O-carboxymethyl Chitosan O-CMC under mild conditions, resulting in strong cross-linking. PEG significantly improved the films’ flexibility, water vapor resistance, and antibacterial properties. Films with 6% PEG showed the best performance in terms of water vapor resistance and antibacterial activity, especially against Staphylococcus aureus. The study concluded that O-CMC–PEG films with optimized PEG content are highly effective for preserving fruits and vegetables, enhancing shelf life and food safety.
*DPFW: Date palm waste, PEG: Poly ethylene glycol, Cs: Chitosan
Challenges and Limitations in PEG Applications
Limited biodegradability
One of the significant challenges associated with the use of PEG across various industries, particularly in the food and pharmaceutical sectors, is the concern regarding its biodegradability. While PEG is typically degraded within the body and is highly beneficial in pharmaceutical and medical applications, its extensive use in the production of packaging materials can lead to environmental accumulation and incomplete degradation [132]. These concerns arise from the fact that in certain packaging applications, especially in food industry, prolonged non-degradability of PEG may have adverse environmental impacts.
Adverse Health Effects
Another concern is the potential health problems associated with the use of PEG in food and pharmaceuticals. While PEG is widely regarded as a safe and non-toxic polymer, and is commonly found in many health products and pharmaceuticals, excessive consumption may lead to digestive problems or allergic reactions [133]. In food packaging, it is important to be careful with the amount of PEG used to avoid potential problem.
Challenges in Fine-Tuning PEG Levels
Another challenge associated with the use of PEG in food packaging is the need to fine-tune the amount of this polymer used. While PEG can significantly enhance the mechanical and antibacterial properties of packaging films, excessive use may adversely affect the taste, texture, and overall quality of the food products [134]. This is particularly critical for foods that are sensitive to physical and chemical changes, where even minor alterations can impact consumer acceptability.
Conclusion and future perspective
Polyethylene glycol (PEG) has established itself as a transformative material with vast potential across multiple industries, particularly in biomedicine and food packaging. In the biomedical field, PEGylation has revolutionized drug delivery systems by enhancing the efficacy, stability, and half-life of biologic drugs, while simultaneously reducing immunogenicity and adverse side effects. PEG-based hydrogels, with their stimuli-responsive and controlled-release capabilities, have shown remarkable promise in targeted therapies for cancer, autoimmune diseases, and wound healing. These advancements highlight PEG’s versatility and its ability to address some of the most pressing challenges in modern medicine.
In the food industry, PEG has emerged as a key player in the development of innovative packaging solutions. Its unique properties, such as moisture retention, antibacterial activity, and mechanical flexibility, have significantly contributed to extending the shelf life and improving the safety of food products. From antimicrobial films to nanocomposites, PEG-based materials have opened new avenues for sustainable and efficient food preservation.
Despite these advantages, PEG is not without limitations. One of the most significant challenges is its limited biodegradability, which raises concerns about environmental accumulation, particularly in packaging applications. Additionally, while PEG is generally regarded as safe, its potential accumulation in the human body and associated health risks necessitate further investigation. Fine-tuning PEG’s molecular weight and optimizing its properties for specific applications remain critical areas for future research.
Looking ahead, the development of biodegradable PEG alternatives and the optimization of PEG’s molecular weight and properties will be essential to overcoming these challenges. Furthermore, interdisciplinary research focusing on the long-term environmental and health impacts of PEG will be crucial for ensuring its sustainable use. By addressing these limitations, PEG can continue to drive innovations that improve human health, enhance food safety, and contribute to environmental sustainability. As research in this field progresses, the potential for PEG to revolutionize these critical industries remains vast and promising, paving the way for next-generation materials that balance performance, safety, and sustainability.
Statements and Declarations
Authors' contributions
Abolfazl Jahani, Conceptualization, Investigation, Writing - Original Draft; Hoda Nassira, Methodology, Validation, Writing - Review & Editing, Supervision.
Competing Interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Data availability
Not applicable.
Funding
This paper received no external funding.
Authors’ Information
Abolfazl Jahani—Polymer Chemistry Research Laboratory, Faculty of Chemistry and Petroleum Science, Shahid Beheshti University, Tehran, Iran;
Hoda Nassira—Polymer Division, Department of Chemistry, Faculty of Science, University of Zanjan, Postal Code: 45371–38791, Zanjan, Iran;


.tif)
 - Copy copy.png)