Visual Abstract
Abstract
In the ever-evolving field of pharmaceutical research, the search for innovative drug delivery systems persists to meet the growing demand for more effective, targeted, and patient-friendly therapeutic interventions. Among the diverse compounds explored for drug delivery, tannic acid (TA)—based materials have emerged as an up-and-coming platform. TA, a polyphenolic compound abundant in various plant sources, has attracted attention due to its unique structural characteristics and versatile properties, rendering it a compelling candidate for drug delivery applications. Its versatility spans various pharmacological functions, including anti-toxicity, anti-cancer properties, anti-allergic and anti-inflammatory traits, anti-microbial and anti-viral effects, wound healing capabilities, and more. This article delves into the multifaceted world of TA, covering its structural attributes, properties, and remarkable applications in biomedicine. Beyond its utility as a component in consumables, TA's role in the green synthesis of nanosized metal particles and its potential in drug delivery are discussed. Throughout this exploration, we emphasize the innate capacity of TA-based supramolecular materials to craft intricate structures, offering many innovative medical prospects. This review aims to provide insights into TA's potential to generate pioneering medical solutions to meet the ever-evolving demands of healthcare and biomedicine.
Introduction
In the landscape of pharmaceutical research, the search for innovative drug delivery systems is nonstop, driven by the ever-increasing demand for more effective, targeted, and patient-friendly therapeutic interventions. Novel drug delivery systems represent a dynamic and constantly evolving field of study that seeks to revolutionize the way medications are administered, with the overarching goal of optimizing drug efficacy, minimizing side effects, and enhancing patient compliance [1–8].
Among the different compounds investigated for this drug delivery purposes, tannic acid (TA)-based materials have emerged as a promising platform with vast potential. TA, a polyphenolic compound found in abundance in various plant sources, has captivated researchers due to its unique structural features and versatile properties, making it an intriguing candidate for drug delivery applications. Its pharmacological versatility spans domains such as anti-toxicity, anti-cancer properties, anti-allergic and anti-inflammatory characteristics, anti-microbial and anti-viral effects, wound healing capabilities, and more [9–12].
Although there are some published review articles on the use of TA in biomedical applications [13–15], this article is intended to focus on the application of TA in drug delivery systems. Therefore, we highlight the world of TA, exploring its diverse facets, from its structural and property attributes to its remarkable biomedical applications. Beyond its potential as an ingredient in consumables, TA’s role in the green synthesis of nanosized metal particles as well as drug delivery is covered. Throughout this exploration, we emphasize the intrinsic capability of TA-based supramolecular materials to craft intricate structures, offering a plethora of innovative medical prospects. This review aims to provide an understanding of TA’s potential to generate groundbreaking medical solutions that can meet the evolving demands of healthcare and biomedicine.
Structure and properties
TA is well-known for its complex molecular structure, characterized by a profusion of phenolic hydroxyl groups. These distinctive features render it amenable to forming multifaceted interactions with a wide range of substances. Its ability to self-assemble, precipitate, and chelate metal ions, combined with its excellent biocompatibility, all make it an exceptional choice for constructing drug delivery carriers. Understanding the structure and properties of TA is pivotal to unlocking its full potential in drug delivery systems.
Tannins, which are polyphenolic phytochemicals, are non-toxic and environmentally friendly compounds commonly found in various beverages and foods, including green tea, raspberries, and black-eyed peas [16]. Tannins can be broadly categorized into two types: condensed tannins (non-hydrolyzable) and hydrolyzable tannins [17]. As a polyphenol, tannin possesses a wide range of medicinal, therapeutic, and healing properties, making it a valuable antioxidant. Consequently, it exhibits various pharmacological benefits, including anti-toxic, anti-cancer, anti-allergic, anti-inflammatory, deworming, antimicrobial, antiviral, wound healing, and dysentery healing properties [18].
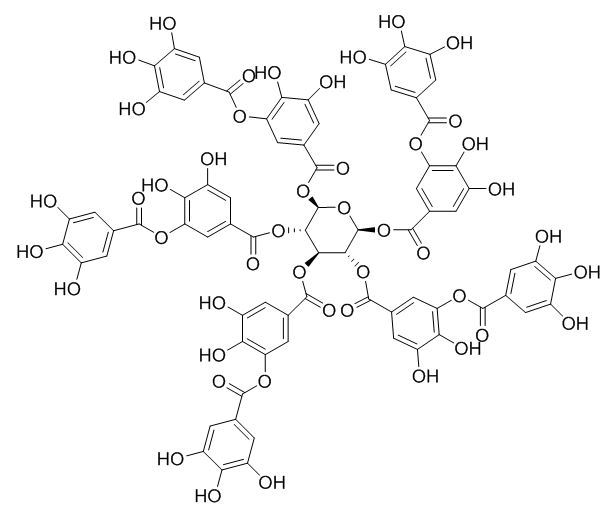
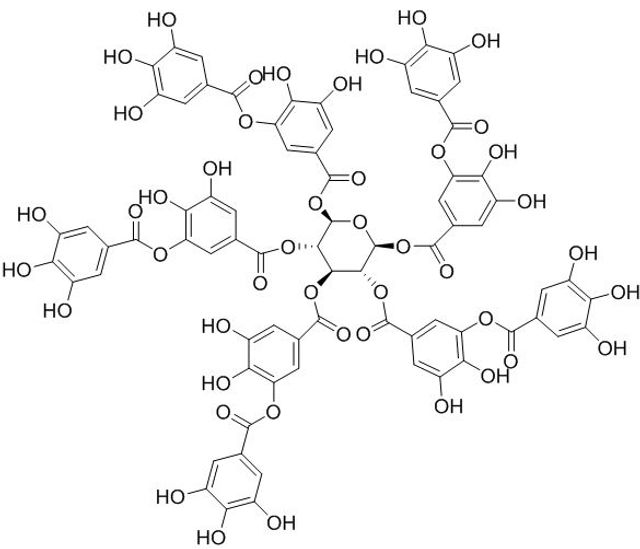
TA is a natural tannin derived from a phenolic acid group, consisting of a central glucose unit and ten gallic acid molecules connected to it, as shown in Figure 1 [19]. It is a water-soluble polyphenolic compound that is recognized to be safe by the Food and Drug Administration. TA molecule contains myriad active phenolic groups with facile conversion into poly (tannic acid) (PTA), which can adhere to a variety of molecules via covalent and/or non-covalent bonds [20].
Applications in green synthesis of metal nanostructures
In the field of metal nanoparticle synthesis, traditional chemical methods have long been the standard, often relying on a wide array of chemical agents, including reducing agents, capping agents, and organic solvents. These methods generally involve the use of toxic or hazardous chemicals, such as hydrazine, sodium borohydride, or various organic ligands, which not only pose risks to the environment but also raise concerns about the safety of researchers and operators. Moreover, the production of metal nanoparticles through chemical routes often demands intricate controls of reaction conditions, temperature, and pressure, which can be resource-intensive and time-consuming. These limitations have prompted the exploration of more sustainable, eco-friendly, and safer alternatives for synthesizing metal nanostructures [21–24].
This innovative approach leverages the unique properties of bioactive compounds, plant extracts, and microorganisms to reduce and stabilize metal ions, facilitating the formation of nanoparticles. Unlike traditional chemical methods, green synthesis offers a safer, cost-effective, and environmentally friendly route to crafting metal nanostructures. These green approaches not only reduce the risk of toxic exposure but also implement the principles of green chemistry, which prioritize the minimization of hazardous substances and the promotion of sustainability. Moreover, green synthesis techniques are often simple, efficient, and amenable to large-scale production, making them attractive for various applications across industries, from nanomedicine to catalysis, and environmental remediation [25, 26]. This section introduces the applications of green synthesis in the creation of metal nanostructures, emphasizing their potential to revolutionize the field by addressing the shortcomings of traditional chemical methods.
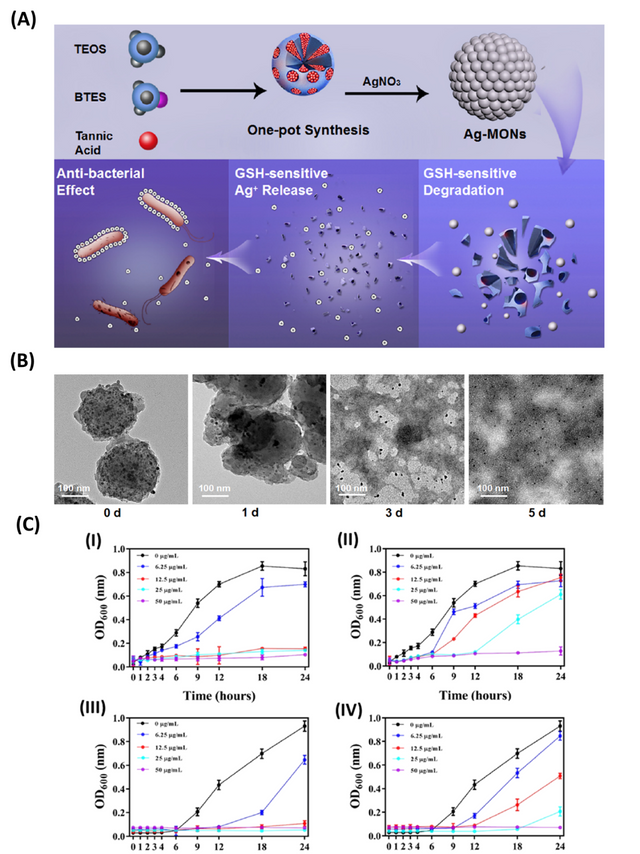
TA has been employed as a reducing agent to produce metallic nanoparticles with strong antibacterial activity using a one-pot environmentally-friendly process. In one report, mesoporous organosilica nanoparticles (MONPs) decorated with Ag-NPs were synthesized by the reduction of silver ions in the presence of TA (Figure 2). This nano platform showed strong antibacterial activity and biodegradability. These nanoparticles displayed a matrix degradation mechanism with the incorporated silver ions released in response to glutathione. The Ag-MONPs-TA nanoclusters exerted a strong antibacterial activity against E. coli and S. aureus with biocompatibility higher than the individual components [27].
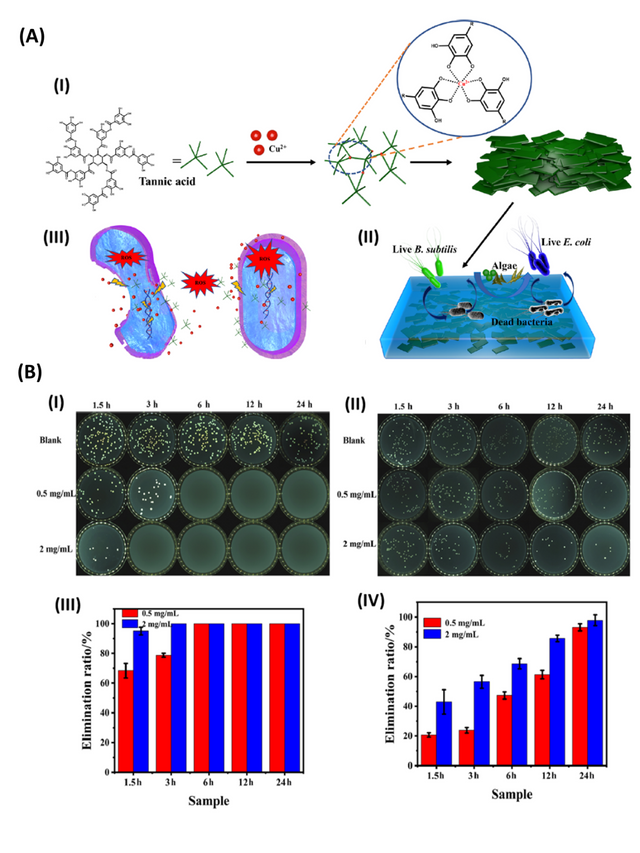
In another work, metal-organic nanosheets were synthesized from copper and TA (Cu-TA), as shown in Figure 3. The synthesized nanoparticles exhibited excellent antibacterial and antifouling properties. Specifically, Cu-TA nanosheets with concentrations of 0.2 and 0.5 mg/mL demonstrated a strong antibacterial activity, where the killing rates against E. coli and B. subtilis bacteria reached almost 100% after 24 h of exposure. Morphological and elemental distribution studies on bacteria revealed that Cu-TA compromised the integrity of the cell wall of the bacteria, thus hindering its ability to grow and proliferate. The Cu-TA nanosheets in combination with acrylic resin were shown to exhibit excellent antibacterial activity against E. coli and B. subtilis bacteria as well as algicidal properties [28].
Application in Drug Delivery
Drug delivery systems are meticulously engineered to achieve one of two primary goals: precise targeting of a specific location within the body or the controlled release of therapeutic agents at a designated site. In doing so, these systems offer a sophisticated solution to circumvent the longstanding limitations associated with conventional free-form drug administration. These limitations encompass factors such as chemical instability, limited bioavailability, reduced solubility, and the risk of systemic cytotoxicity [29–33].
The pioneering drug delivery systems encompass a wide array of approaches, from utilizing advanced materials to engineering intricate nanostructures. They offer the potential to transform the way we treat a vast range of medical conditions, addressing challenges that traditional drug delivery methods often cannot. Novel delivery systems are designed to enhance the pharmacokinetics and pharmacodynamics of drugs, ensuring that they reach their intended sites of action with precision and efficiency [34, 35].
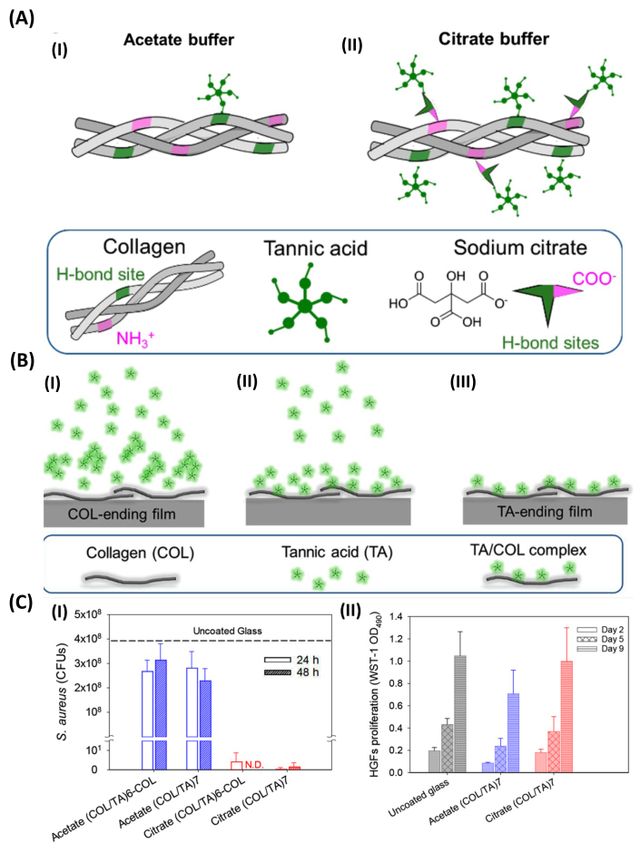
The prevalence of multidrug-resistant antimicrobial species like bacteria has heightened the demand for more effective antibacterial agents with direct bactericidal actions [33]. In a study, conducted by Boulmedais et al., the deposition of polyelectrolyte multilayers using the layer-by-layer method was investigated as a well-established technique for creating biocompatible and antibacterial coatings to prevent implant-associated infections (Figure 4) [36]. Numerous layer-by-layer films have been developed to possess antiadhesive and antibacterial properties, and their effectiveness is influenced by factors like the incorporated compounds, deposition conditions, and post-buildup treatments. TA, a natural polyphenol known for its ability to inhibit the growth of various bacterial strains, was used in this work to create TA/collagen layer-by-layer films in either acetate or citrate buffers at pH 4. Surprisingly, the choice of buffer not only affected the physicochemical properties of the films but also influenced their antibacterial characteristics. Under physiological conditions, both types of TA/COL films released similar amounts of TA depending on the last layer, and they exhibited antibacterial properties against Staphylococcus aureus, but only the citrate-built films demonstrated this efficacy. Notably, the citrate-built TA/COL films featured a granular surface topography, which enhanced their release-killing effect, while remaining non-cytotoxic to human gingival fibroblasts.
The study emphasized a comprehensive evaluation of the physicochemical parameters governing film buildup and antibacterial performance, particularly focusing on the different complexation strengths between TA and COL in the presence of the two buffers. This research represents a significant step towards utilizing polyphenols like TA as antibacterial agents when incorporated into LbL films, offering potential applications in preventing implant-related infections [36].
Cancer stands as a formidable global health challenge and ranks as the second leading cause of mortality in the United States. Despite relentless endeavors to discover effective anti-cancer treatments, the progress has been modest [37, 38]. Notably, while traditional cancer treatment methods, such as surgery, chemotherapy, and radiotherapy (RT), have witnessed significant technological advancements in recent years, they often fall short in terms of efficacy and are plagued by adverse side effects. These methods, when employed in isolation, frequently prove insufficient, particularly when dealing with tumors intertwined with normal tissues. Additionally, multidrug resistance (MDR) resulting from repeated chemotherapy and the insensitivity of hypoxic cancer cells to ionizing radiation contribute to treatment failures post-chemotherapy and RT [39, 40].
Two key chemotherapeutics, doxorubicin (DOX) and paclitaxel (PTX) have shown exceptional anti-tumor efficacy against various solid tumors, including breast cancer. PTX, a hydrophobic anti-cancer drug, induces apoptosis through microtubule disruption, while DOX, a hydrophilic anti-cancer drug, inhibits DNA synthesis and subsequently triggers tumor cell apoptosis. For instance, Huang et al. developed PTX nanocrystals (PNC) coated with PTA. When subjected to laser illumination, PNC-pTA exhibited enhanced cytotoxicity against the 4T1 cell line compared to cases without laser irradiation. Animal experiments confirmed that these nanoparticles exhibited a mild photothermal effect within the body and demonstrated the strongest tumor-inhibitory effect under laser irradiation. This research underscored the potential of PNC-pTA as a promising nano platform for combined chemo-photothermal therapy [38, 41].
In a similar vein, TA-PTX nanoparticles and PTX-loaded TA/poly(N-vinylpyrrolidone) nanoparticles displayed significant tumor-suppressive effects in xenograft breast tumor models. The results highlight these nano-formulations as a pivotal reference for the development of therapeutic formulations with substantial and enhanced effects in cancer treatment [42, 43]. PTX delivery by TA-based nanoparticles exhibited heightened anti-cancer properties, as evidenced by decreased proliferation, reduced clone formation, and an effective deterrent against metastasis, as reflected in diminished migration and invasion. A study modified mesoporous silica nanoparticles (MSN) by coating the surface with PTA and HER2 antibodies, creating reversible pH-responsive and targeted HER2 nanoparticles with high drug loading [44]. Remarkably, both in vitro and in vivo experiments demonstrated the reversible pH-responsive release attributes of these nanocarriers and their capability to effectively suppress tumor growth with minimal side effects. These nanocarriers displayed superior in vitro cytotoxicity, reusability, and minimal in vivo myocardial damage, collectively resulting in significantly enhanced therapeutic efficacy and an exceptional anti-tumor impact. Recent advances in nanocarrier synthesis enable the incorporation of dual drug delivery systems, encapsulating two drugs to bolster therapeutic outcomes.
In a study, He and colleagues [45] developed a TA/exendin–4/Fe3+ nanoparticle system to provide sustained release of exendin–4. Efficacy studies conducted in a mouse model of type 2 diabetes proved that the amount of exendin–4 released by nanoparticles was not only higher than a free exendin–4 injection but also showed that this nanocarrier can quickly dissolve after a single injection. The blood glucose level is reduced to the normal range by intraperitoneal administration, and the level remains the same for three days. In drug systems, TA could play various roles such as hydrotrope-like solubilizing agent and transfer agent which facilitates the aqueous solubility of hydrophobic drugs and the transformation of drugs into cells in many cell lines [45].
In another study, In vitro drug release behavior demonstrated that at pH 7.4, Fe3O4@SiO2@TA has the least drug leakage, which can reduce the adverse effects on normal tissues [46]. On the other hand, at pH 5, the drug release is significantly higher, which can enhance the cytotoxicity of cancer tissues. Also, the cell cytotoxicity test (MTT) proved that TA-based nanocarrier has higher cytotoxicity against MCF7 cell lines than free medications which indicated this nanocarrier can be a potential cancer tissue targeting [46].
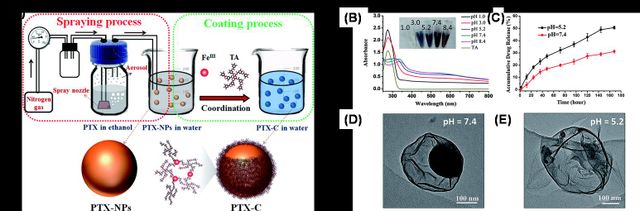
In another study, a novel one-step fabrication process to obtain PTX nanoparticles (PTX-NPs) encapsulated by thin-film (PTX-C) was reported with well-controlled morphology and size [47]. The PTX-NPs were fabricated by aerosol generators, and the prepared PTX-NPs were simultaneously further coated by coordination of polyphenol TA and FeIII. The resulting PTX-C nanodrugs were not only stable in water but also pH-responsive for drug release (Figure 5). The highly efficient pH-responsive drug release, uptake by cells, and localization at lysosomes contribute to PTX-C serving as an excellent anti-tumor nano-drug. The IC50 values of PTX-C are similar to or significantly lower than the values of PTX and ABI-007 in 6 different cancer cell lines. PTX-C leads to a 69.2% tumor growth inhibition rate, compared with 14.7% and 99.9% in the cases of PTX and ABI-007, respectively.
Conclusion and Outlook
One of the prominent areas of focus within drug delivery is the development of biomaterial-based, where natural and synthetic materials are ingeniously harnessed to encapsulate, protect, and transport therapeutic agents to specific tissues or cells [48, 49]. These materials may include biocompatible polymers, lipids, or even natural compounds like TA. By combining pharmaceutical agents with these biomaterials, researchers can achieve prolonged release, targeted delivery, and enhanced bioavailability, all while mitigating potential toxicities.
In conclusion, the review consolidates the key findings and insights regarding the use of TA-based materials in drug delivery applications. Additionally, it provides an outlook on the future of this exciting field, suggesting potential areas of growth and development. As pharmaceutical research continues to evolve, TA-based materials offer a compelling avenue to explore, promising to deliver innovative and effective drug delivery solutions that can address the unmet needs of patients and healthcare systems.
Statements and Declarations
Authors' contributions
All authors contributed to drafting, and revising the paper and agreed to be responsible for all aspects of this
work.
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Data availability
Not applicable.
Funding
No funding was received to support this work.
Authors’ Information
Rasoul Esmaeely Neisiany—Department of Polymer Engineering, Hakim Sabzevari University, Sabzevar, 9617976487, Iran; Biotechnology Centre, Silesian University of Technology, Krzywoustego 8, 44–100, Gliwice, Poland;
Mohammadsaeid Enayati—Center of Experimental Orthopaedics, Saarland University Medical Center, Kirrbergerstr. Bldg 37, 66421 Homburg/Saar, Germany;
Oisik Das—Department of Civil, Environmental, and Natural Resources Engineering, Luleå University of Technology, 97187, Lulea, Sweden.


.jpg)
 - Copy copy.png)