Visual Abstract
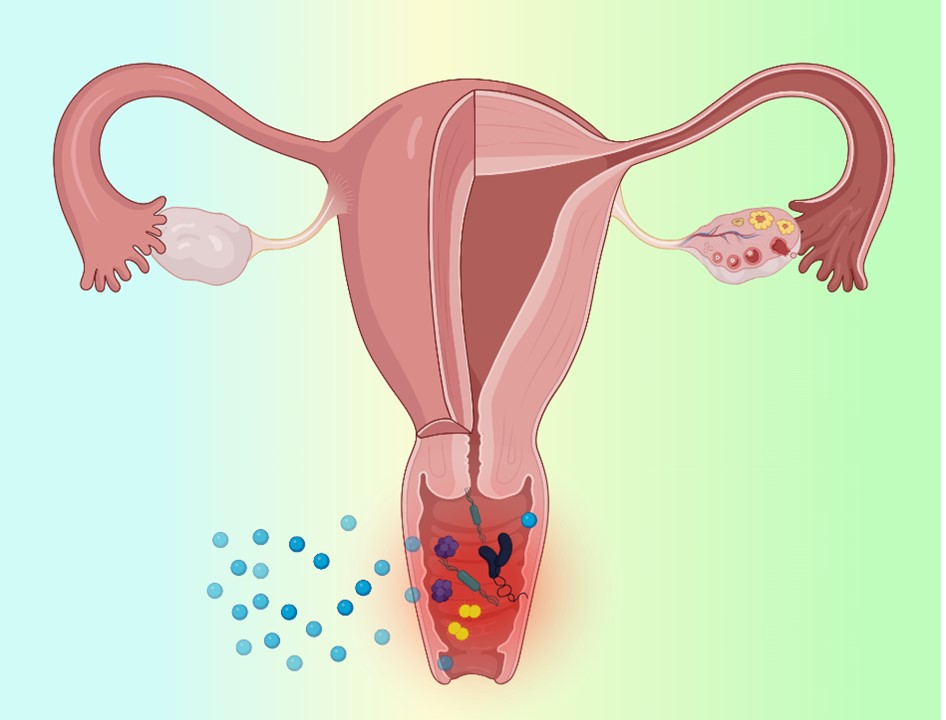
Abstract
The vaginal route offers unique advantages for localized and systemic drug delivery, but efficacy is limited by biological barriers including mucus, epithelium, immune cells, and microbiota. Prevalent microbe-associated infections like bacterial vaginosis, candidiasis, and trichomoniasis often recur due to treatment failures. Drug delivery via nanomaterials and hydrogels provides opportunities to overcome limitations through platforms that modulate drug release kinetics, mucoadhesion, mucus penetration, and intrinsic antimicrobial properties. This review discusses biological barriers, prevalent vaginal infections, and nanoscale delivery systems including nanoparticles, liposomes, hydrogels, and inorganic materials. Surface engineering allows the customization of nanoparticles for mucoadhesive or mucus-penetrating properties. Liposomes can fuse with cell membranes for intracellular delivery. Hydrogels provide sustained drug release. Inorganic nanomaterials exhibit inherent antimicrobial effects. These nanosystems offer targeted, sustained drug delivery to improve treatment outcomes for vaginal infections. Further research is warranted to establish clinical efficacy and safety.
Introduction
The vagina serves as an important route for localized and systemic drug delivery due to its large surface area, rich blood supply, and avoidance of first-pass metabolism [1]. However, the efficacy of vaginal drug delivery is limited by biological barriers like the mucus layer, epithelium, immune system, and microbiome[2,3]. Microbe-associated vaginal infections including bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis are highly prevalent and can lead to complications if untreated[4]. Nanotechnology has enabled the development of drug delivery platforms like nanoparticles and hydrogels to overcome biological barriers and improve vaginal drug delivery [5–7].
In this review, we will discuss the biological barriers, common microbe-associated vaginal infections, and nanoscale drug delivery platforms being explored for improved vaginal drug delivery. The aim is to provide a comprehensive overview of the vaginal environment, infections, and nano-based strategies being developed to enhance treatment. The vagina provides direct access to the local mucosa and rich vasculature for absorption while avoiding first-pass metabolism, making it advantageous for drug delivery. However, the complex vaginal environment provides challenges that must be addressed. We will review the key biological barriers and characteristics of the most prevalent vaginal infections. Finally, we will examine nanotechnology-enabled delivery platforms designed to overcome limitations and provide sustained and targeted drug delivery.
Biological barriers to vaginal drug delivery
The key biological barriers that impact vaginal drug delivery include the mucus layer, epithelium, immune system, microbiome, and physiological variations [8]. The mucus layer traps foreign particles through steric and adhesive interactions [9]. Cervicovaginal mucus coats the vaginal epithelium and acts as a protective barrier against pathogens and foreign particles [10]. The mucins and glycoproteins in mucus provide both a physical and chemical barrier that can inhibit the penetration of drug delivery systems. Mucoadhesive systems can be utilized to increase retention, while mucus-penetrating systems are designed to avoid adhesive interactions and rapidly penetrate mucus [11].
The epithelium restricts paracellular permeability through tight junctions [12]. The vaginal epithelium is made up of stratified squamous epithelial cells covered in mucus [13]. Tight junctions between the epithelial cells limit transcellular and paracellular transport across this layer. Disruption of tight junctions and transport through epithelial cells must be considered for mucosal drug delivery. Epithelial thickness, which varies across the menstrual cycle, also impacts drug absorption [14].
Immune cells can recognize and eliminate foreign particles [15]. The vaginal mucosa contains immune cells like dendritic cells, macrophages, T cells, and neutrophils [16]. These immune cells protect the vagina by recognizing and eliminating foreign materials including pathogens and drug delivery systems [17]. The immune response must be considered as vaginal drug delivery systems will interact with these immune cell populations.
The microbiome inhibits the overgrowth of pathogens but some commensal bacteria impact drug delivery. Lactobacilli represent the dominant vaginal bacteria in reproductive-age women. They promote vaginal health by producing lactic acid to maintain an acidic pH and prevent overgrowth of pathogenic bacteria. Disruption of the microbiome through conditions like bacterial vaginosis reduces natural defenses. However, some microbial enzymes can also impact drug stability and release [18].
Physiological variations related to the menstrual cycle and hormone levels also modulate the vaginal environment [19]. The thickness and properties of the mucus layer vary across the menstrual cycle under estrogen and progesterone influence. Inflammatory cells migrate into the mucosa at different stages of the cycle. These physiological changes impact the vaginal microenvironment and must be factored into the development of drug-delivery systems [20].
Microbe-associated vaginal infections
The major microbe-associated vaginal infections include bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis. Bacterial vaginosis involves a polymicrobial imbalance, commonly with the overgrowth of Gardnerella vaginalis [2]. These Vulvovaginal candidiasis are most often caused by Candida albicans overgrowth [21]. Trichomoniasis is caused by the parasite Trichomonas vaginalis infections are highly prevalent globally and can increase susceptibility to sexually transmitted infections and pregnancy complications if not properly treated [22,23]. Nanotechnology provides opportunities to improve treatment through enhanced mucus penetration, cellular targeting, sustained release, and intrinsic antimicrobial properties [24].
Bacterial vaginosis is characterized by a reduction in beneficial lactobacilli and overgrowth of anaerobic bacteria including Gardnerella vaginalis [25]. Diagnostic criteria include vaginal discharge, elevated pH, amine odor, and microscopic clues [25,26]. Bacterial vaginosis increases susceptibility to sexually transmitted infections including HIV, and has been associated with adverse pregnancy outcomes. Current antibiotic treatment regimens have high recurrence rates. Nanoparticle platforms providing sustained antibiotic release and intrinsic antimicrobial properties may improve bacterial vaginosis treatment.
Vulvovaginal candidiasis is among the most common gynecological infections, affecting up to 75% of women. It is caused by overgrowth of yeasts, mainly Candida albicans. Symptoms include itching, irritation, and vaginal discharge. Predisposing factors include diabetes, antibiotics, hormones, and pregnancy. Azoles are commonly used for treatment, but resistance is increasing. Nanoparticle platforms with mucoadhesive or mucus-penetrating properties can enhance antifungal delivery and improve treatment outcomes[27].
Trichomoniasis is caused by the parasitic protozoan Trichomonas vaginalis. Symptoms include discharge, irritation, and odor [22,28]. It may increase the risk of premature rupture of membranes, preterm birth, and low birth weight infants if acquired during pregnancy. Metronidazole is the drug of choice for treatment, but resistance has been reported. Nanoparticles with high drug loading, sustained release, and intrinsic trichomonacidal activity may help overcome resistance and treatment failures [29].
Delivery Platforms
A range of nanoscale platforms have been explored for improved vaginal drug delivery, including nanoparticles [7,30], liposomes [31], hydrogels [32], and inorganic nanomaterials [2]. Nanoparticles made from polymers like PLGA can be optimized for mucoadhesion or mucus penetration through surface engineering (Table. 1) [33]. For example, chitosan nanoparticles have been shown to exhibit mucoadhesive properties that can increase vaginal retention [34]. Alternatively, PEGylation provides a mucus-penetrating nanoparticle surface [33]. Liposomes fuse with cell membranes for drug delivery and can also exhibit intrinsic antimicrobial effects [31]. For example, liposomes containing essential oils have been shown to have activity against Candida albicans [35].
Type of Formulation | Polymer Used | Disease Type | Drug | Ref |
Conventional liposomes | Phosphatidylcholine | Escherichia coli-related vaginal infections | Azithromycin | [36] |
Chitosan-coated liposomes | Phosphatidylcholine Chitosan | Vaginal inflammation | Resveratrol | [37] |
Chitosan-surface modified PLGA nanoparticles | Poly(lactic-co-glycolic acid) (PLGA | Candida albicans vaginal infections | Clotrimazole | [38] |
Eudragit RL100 nanoparticles coated with HA | Eudragit RL100 HA | Vulvovaginal candidiasis | amphotericin B | [39] |
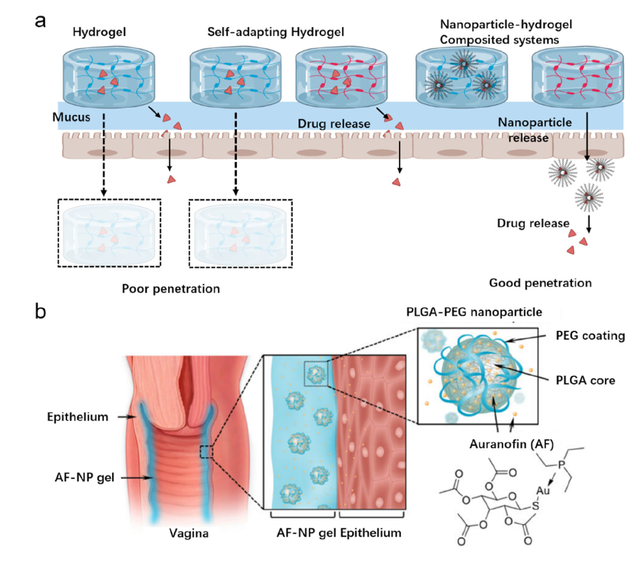
Hydrogels provide sustained drug release and mucoadhesion [32,40]. Thermosensitive hydrogels that gel at body temperature have been designed for vaginal drug delivery. Composite platforms have also been explored, such as nanoparticles encapsulated in hydrogels. This combines the benefits of a hydrogel vehicle with mucus-penetrating nanoparticles for drug release. Figure 1. Shows a schematic illustration of nanoparticles used for drug delivery with potential applications for vaginal infection [41].
Inorganic nanomaterials like silver and zinc oxide have intrinsic antimicrobial properties [2,42]. Silver nanoparticles exhibit broad-spectrum activity through mechanisms such as membrane disruption, metal ion release, and reactive oxygen species generation [43]. They have demonstrated activity against bacteria associated with bacterial vaginosis including Prevotella and Mobiluncus species [44]. Zinc oxide nanoparticles also have antimicrobial effects by inducing reactive oxygen species and disrupting bacterial cell membranes [45]. However, the potential toxicity of inorganic nanomaterials must be carefully evaluated.
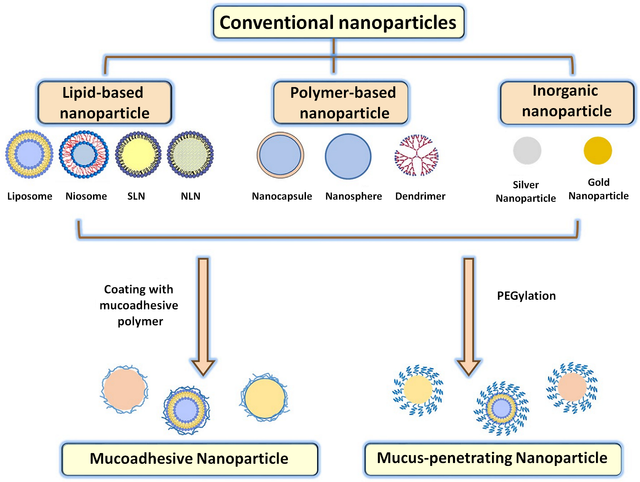
Targeted delivery can be achieved through ligand-receptor interactions [46]. Nanoparticles conjugated with antibodies, peptides, or other targeting moieties can provide improved delivery to specific cell types like epithelial cells or macrophages. This allows for intracellular delivery and higher localized drug concentrations [47].
The nanoscale size also confers key advantages. The small size facilitates penetration through mucus barriers and uptake into cells and tissues. It also provides a high surface area for functionalization with drugs, targeting ligands, and bioactive compounds. Methods like layer-by-layer assembly allow the fabrication of multifunctional nanoparticles. The nanoscale size and tunable properties enable tailored drug delivery [48,49].
Hydrogels have been extensively explored for vaginal drug delivery due to their advantageous physicochemical and biological properties, including mucoadhesion and biocompatibility (Figure 2) [50]. By incorporating responsiveness to pH or temperature, hydrogels can provide controlled drug release tailored to the vaginal environment [51]. However, the large size of conventional hydrogels limits their ability to penetrate vaginal mucus and distribute drugs evenly [32]. To address this, nanoparticle-hydrogel composite systems have been developed that possess the mucoadhesive properties of hydrogels along with the mucus-penetrating abilities of nanoparticles. For example, Eckmann et al. [50] formulated auranofin-loaded nanoparticles embedded within a thermosensitive chitosan hydrogel. This system provided sustained drug release and was able to eliminate Trichomonas vaginalis infections in a mouse model, demonstrating potential for treating bacterial and fungal vaginal infections. The nanoparticle-hydrogel composites combine the strengths of both components, leveraging the mucoadhesion and sustained release of hydrogels along with the mucus penetration and absorptive properties of nanoparticles [50]. This allows improved distribution and retention in the vaginal canal compared to hydrogels alone. The development of nanocomposite systems seeks to overcome the limitations of conventional hydrogels for more effective vaginal therapy.
Conclusion
The vagina provides a promising route for drug administration, but efficacy is limited by biological barriers. Prevalent microbe-associated vaginal infections require improved treatment options. Nanotechnology has enabled the development of diverse platforms that address limitations in vaginal drug delivery through modulation of size, surface properties, drug release, and intrinsic antimicrobial activity[2]. Liposomes [31], nanoparticles [30], hydrogels [32], and inorganic nanomaterials [1] provide opportunities for sustained release, mucoadhesion, mucus penetration, cellular targeting, and intrinsic antimicrobial effects. However, biocompatibility, toxicity, and clinical effectiveness must be evaluated as these platforms progress through preclinical and clinical research. Further research is warranted to advance these platforms through human trials and ultimately provide more effective treatments for debilitating vaginal infections that impact women globally. The integration of nanotechnology and drug delivery has immense potential to overcome biological barriers and provide targeted treatment options for vaginal infections.
Statements and Declarations
Authors' contributions
All authors have the same contribution.
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Data availability
Not applicable.
Funding
No funding was received to support this work.
Authors’ Information
Madineh Moradialvand—Department of Pharmaceutical Engineering, Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran, Iran.
Mina Hoori—MD. Research assistant at Department of Obstetrics and Gynecology, UTHealth Houston, Texas, USA.
Rezvan Hoori—MD. Physician at Department of Obstetrics and Gynecology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.



.jpg)
 - Copy copy.png)


