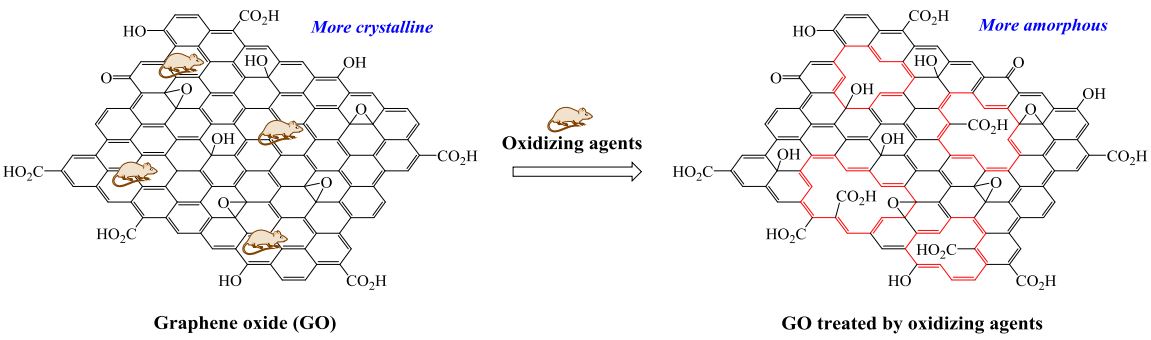
Visual Abstract
Abstract
In this research, the effects of peroxide and persulfate oxidizing agents such as hydrogen peroxide (H2O2), potassium persulfate (K2S2O8), ammonium persulfate ((NH4)2S2O8) and meta-chloroperoxybenzoic acid (mCPBA) on the texture of graphene oxide (GO) are considered by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM) and elemental mapping via energy dispersive X-ray spectroscopy (EDX). According to FT-IR results, GO and GO treated by oxidizing agents contain a layer of carbon atoms that decorated by oxygen-containing groups. Based on XRD data, a phase transition from more crystalline graphene oxide to more amorphous state is observed where GO is treated by oxidizing agents. It seems that oxidizing agents can create and enlarge the hole defects in graphene oxide. Significant changes in the morphology of the GO after treatment by oxidizing agents are observed via the FESEM micrographs. The value of the C/O atomic ratio for the GO treated by mCPBA is 1.23. This compound show the higher degree of oxidation than GO and GO treated by other oxidants. The corresponding value for the prepared GO is 1.94.
Introduction
Oxidation reactions have an important role in organic chemistry so significant progress has been achieved within this area in recent decades [1]. There are several different definitions for oxidation, i.e. loss of electrons, increase in oxidation state, loss of hydrogen, or gain of oxygen [2]. An oxidizing agent is a substance that is capable of causing oxidation [3]. Oxidizing agents are important in many chemical processes including combustion, metabolism, and corrosion [3]. They are also commonly used in industrial processes such as in the production of fuels, purification of water, bleaching of fabrics, vulcanization of rubber, and storage of energy in batteries [3, 4].
Hydrogen peroxide (H2O2) is an oxidizing agent that is commonly found in cosmetics, bleaching agents, toothpaste and detergents [5]. It is medically used as an antiseptic and antibacterial agent for disinfection and wound irrigation [5]. Also, it has been used in dentistry as a mouthwash to reduce plaque and improve recovery after oral surgery [5, 6]. This compound reacts on contact with oxidizable organic materials, metals, and alkaline solutions by producing free hydroxyl radicals, which react with lipids, proteins, and DNA [5]. Aromatic aldehydes can be transformed into phenols by oxidizing with H2O2 in acidic methanol [7]. Alkenes and α,β-unsaturated acids have been epoxidized with H2O2 in a catalytic system [7]. Vicinal diols have been prepared from alkenes by oxidizing with H2O2 in the presence of catalysts [7]. H2O2 has also been used to oxidize alcohols and amines by employing catalysts [7].
Peroxycarboxylic acids such as meta-chloroperoxybenzoic acid (mCPBA) are among the most powerful organic peroxide oxidizing agents [8]. The main industrial uses of these compounds are in the manufacture of epoxides, synthetic glycerol, and epoxy resins [8]. They also have been used as disinfectants, fungicides, bleaching agents, and shrink-proofing wool [8]. Typical reactions include epoxidation and hydroxylation of alkenes, oxidation of sulfides to sulfoxides or sulfones, transformation of ketones to esters or lactones, conversion of aldehydes to carboxylic acids, and oxidation of amines to amine oxides [7, 8].
Persulfates such as potassium persulfate (K2S2O8) and ammonium persulfate ((NH4)2S2O8) are often used in environmental applications such as soil and groundwater remediation [9], but also in other applications such as chemical syntheses, electronics, and as hair dyes and bleaching agents in pharmaceuticals and cosmetics [10]. They are powerful oxidants capable of oxidizing almost all organic compounds [11]. As they can degrade a wide range of organic pollutants, some reports promote the persulfate-based oxidation process as a viable alternative to hydrogen peroxide-based processes in water treatment [12–14].
Graphene oxide (GO) is a single layer of graphite oxide that is exfoliated from this compound [15]. Graphite oxide can be prepared by the intercalation and oxidation of graphite powder [16]. For this purpose, graphite powder is chemically reacted with acids (HCl, H2SO4, and HNO3, etc.) followed by the intercalation of alkali metals (KClO3, KMnO4, NaNO3, etc.) [16]. So many methods have been widely developed in the past decades for the preparation of GO, but the Hummers' method is the most widely used one nowadays [17]. The Hummers' method involves the treatment of graphite with potassium permanganate (KMnO4) and sulfuric acid (H2SO4) [18]. Also, this method has been modified to improve the yielded GO (oxidation degree) and/or to speed up the process [17]. The modified Hummers' method requires H2SO4, KMnO4, H2O, and H2O2 for GO formation [17]. The prepared GO mainly consists of individual sheets of graphene with attachments of oxygen functional groups (hydroxyl, epoxy, carbonyl, and carboxyl) [16].
GO has been explored in a wide range of applications such as energy generation/storage, electronic and photonic devices, optical devices, clean energy, chemical/biosensors, and drug delivery [16]. Moreover, the chemical functionalization of GO by noncovalent and covalent approaches is very attractive for synthetic chemists [15]. Using oxidizing agents as the oxidant [19, 20], initiator [21], cross-linker [22], matrix modifier [23], and catalyst [24] in many synthetic reactions is unavoidable. Therefore, it is important to investigate the effects of oxidizing agents on the texture of graphene oxide which is the subject of this study. To clarify the importance of this issue, some recent publications are mentioned in the following.
El-Sheikh reported the effect of oxidation of multi-walled carbon nanotubes (MWCNTs) with various oxidizing agents such as HNO3, H2O2, and (NH4)2S2O8 [25]. Peng and Liu studied the effects of H2O2 oxidation on the morphology and structure of the MWCNTs [26]. Teoh et al. investigated the evaluation of various oxidizing agents including HNO3, H2SO4, HNO3/H2SO4, and KMnO4 to assess their effectiveness on covalent functionalization of MWCNTs [27]. Zhang et al. reported the systematic investigation of the effect of chemical oxidation on the structure of single-walled carbon nanotubes (SWCNTs) by using different oxidants including HNO3, HNO3/H2SO4, and KMnO4 [28]. Kashif et al. investigated the effect of KMnO4 on graphene oxide (GO) morphological and structural properties [29].
In the current research, the effects of H2O2, K2S2O8, (NH4)2S2O8, and mCPBA on the functional groups, crystallinity, morphology, and degree of oxidation of GO are considered by FT-IR, XRD, FESEM, EDX, and elemental mapping techniques. We report the first comparison between GO and GO treated by peroxide and persulfate oxidizing agents. The findings of this manuscript help researchers choose the appropriate oxidizing agent and use it to optimize the performance of GO in organic synthesis.
Materials and Methods
Materials and Characterization
The fine powder of extra pure graphite and all other chemicals used in this study (sulfuric acid 98%, potassium permanganate, sodium chloride, hydrochloric acid 37%, dichloromethane, sodium bicarbonate, hydrogen peroxide 30%, potassium persulfate, ammonium persulfate, and meta-chloroperoxybenzoic acid 75%) were obtained from Merck.
The FT-IR spectra were recorded using KBr pellets on PerkinElmer (RXI model) Fourier transform infrared spectrophotometer. The XRD measurements were performed using Bruker (D8-Advance) X-ray diffractometer with CuKα radiation. The FESEM images and elemental mapping patterns were taken by a TESCAN MIRA III Field Emission Microscope equipped with an EDS system for elemental analysis.
Experimental
GO was prepared by oxidation of graphite in the modified Hummers method [30] with minor changes. First, graphite (0.5 g) was ground with NaCl (2.5 g, 42.779 mmol). Then, NaCl was dissolved in deionized water and removed by filtration. The remaining graphite was stirred in H2SO4 98% (11.5 mL) for 12 h. Thereafter, KMnO4 (2.5 g, 15.819 mmol) was gradually added while keeping the temperature below 20 °C by an ice bath. The resulting mixture was stirred at 40 °C for 1.5 h. Next, deionized H2O (22.5 mL) was added and the mixture was heated at 55 °C for 0.5 h. The reaction was terminated by the addition of deionized H2O (70 mL) and H2O2 30% solution (5 mL). The mixture was filtrated and washed several times with HCl aqueous solution of 5% and deionized water. The final product was exfoliated by ultrasonication and dried at room temperature for 24 hours.
For treatment of GO with hydrogen peroxide, GO (0.01 g) was added to H2O2 solution 30% (10 mL) and stirred in an oil bath at a pre-set temperature of 80 °C for 24 h. After cooling, the resulting mixture was filtrated and washed several times with deionized water. The final product was dried at room temperature for 24 hours.
For treatment of GO with potassium persulfate, GO (0.01 g) was added to the solution of K2S2O8 (0.125 mmol) in deionized water (5 mL) and stirred in an oil bath at a pre-set temperature of 80 °C for 24 h. After cooling, the resulting mixture was filtrated and washed several times with deionized water. The final product was dried at room temperature for 24 hours.
For treatment of GO with ammonium persulfate, GO (0.01 g) was added to the solution of (NH4)2S2O8 (0.125 mmol) in deionized water (5 mL) and stirred in an oil bath at a pre-set temperature of 80 °C for 24 h. After cooling, the resulting mixture was filtrated and washed several times with deionized water. The final product was dried at room temperature for 24 hours.
For the treatment of GO with meta-chloroperoxybenzoic acid, GO (0.01 g) was added to the solution of mCPBA (0.125 mmol) in dichloromethane (5 mL) and stirred at room temperature for 24 h. The resulting mixture was filtrated and washed several times with dichloromethane, aqueous solution of sodium bicarbonate 10%, and deionized water. The final product was dried at room temperature for 24 hours.
Results and Discussion
For the preparation of GO in Hummers' method, NaNO3 and KMnO4 dissolved in concentrated H2SO4 have been used to oxidize graphite [31]. This method suffers from several defects including toxic gas generation (NO2 and N2O4), residual nitrate, and low yield [32]. In this research, GO is prepared by oxidation of graphite in modified Hummers' method [30]. In this method, KMnO4 and concentrated H2SO4 are used for oxidation of graphite. The sulfuric acid is the concentrated acidic medium that intercalates the graphite and expands it, and potassium permanganate is the oxidant [17]. The main role of H2O2 in modified Hummers' method is to remove the excessive KMnO4 or in other words to stop the reaction [33, 34].
In the following, the effects of peroxide and persulfate oxidizing agents on the texture of GO are considered by FT-IR, XRD, FE-SEM, EDX, and elemental mapping techniques. For this purpose, a specific amount of GO is treated by the aqueous solution of H2O2, K2S2O8, and (NH4)2S2O8 at 80 °C. This temperature for the treatment of GO is chosen to work with a temperature higher than the routine temperatures of GO preparation (i.e. 25–60 °C [35]). As mCPBA is insoluble in water and sensitive to heat, the treatment of GO by this oxidizing agent is performed in dichloromethane at room temperature.
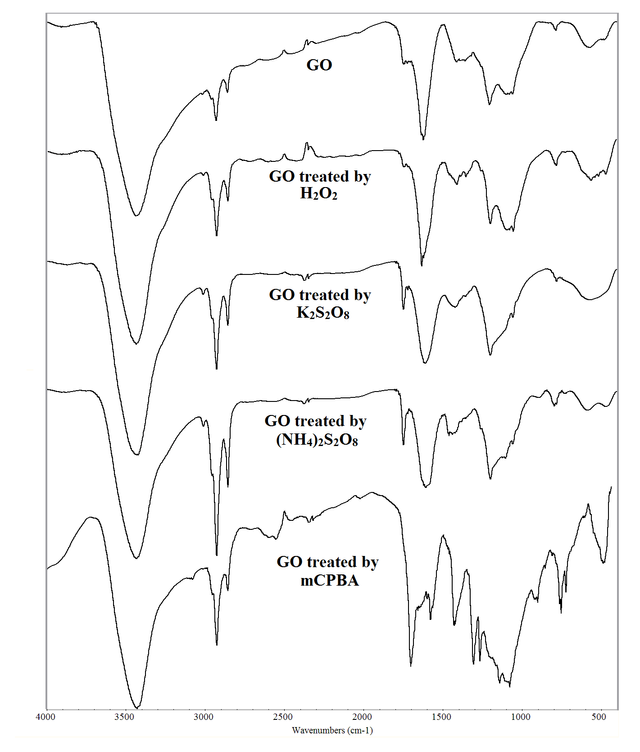
FT-IR spectra of GO and GO treated by oxidizing agents are depicted in Figure 1. Also, frequencies for various modes of vibrations are collected in Table 1. GO contains a layer of carbon atoms that is decorated by oxygen-containing groups such as C-O, C-O-C, C = O, and -OH. This result agrees with that reported in the literature for GO [36, 37]. The broad bands observed at 3000 to 3700 cm–1 with the maxima at 3434 cm–1 for GO and around 3420–3434 cm–1 for other oxidized samples are attributed to the O-H stretching vibrations. The bands appeared at 1358 cm–1 for GO and around 1352–1354 cm–1 for other oxidized samples assigned to the O-H bending vibrations. These bands are the result of the presence of hydroxyl groups within the structures. The bands located at 1742 cm–1 for GO and around 1700–1742 cm–1 for other oxidized samples are assigned to the C = O stretching vibrations, corresponding to the carboxyl groups on the edges of the layer planes or conjugated carbonyl groups. The bands seen at 1406 and 1618 cm–1 for GO and around 1410–1458 and 1576–1630 cm–1 for other oxidized samples are ascribed to the C = C stretching vibrations, confirming that the C = C bonds remain after treatment of GO by oxidizing agents. The bands found at 2854, 2926, and 3006 cm–1 for GO and around 2854–3078 cm–1 for other oxidized samples are assigned to the C-H stretching vibrations, suggesting the presence of both sp3 and sp2 carbon atoms that are created during the oxidation process. The bands centered at 1054, 1096, and 1202 cm–1 for GO are assigned to the stretching vibrations of C-OH, C-O, and C-O-C groups due to phenol, carboxylic acid, ether, and epoxide functionalities. These bands can be observed in the more oxidized samples at around 1052–1138 cm–1.
Vibration mode | GO | GO treated by H2O2 | GO treated by K2S2O8 | GO treated by (NH4)2S2O8 | GO treated by mCPBA |
S(O-H) | 3434 | 3434 | 3420 | 3434 | 3426 |
S(C-H) | 3006, 2926, 2854 | 3008, 2924, 2854 | 3010, 2926, 2854 | 3008, 2924, 2854 | 3078, 2924, 2854 |
S(C = O) | 1742 | 1742 | 1746 | 1746 | 1700 |
S(C = C) | 1618, 1406 | 1630, 1410 | 1608, 1420 | 1604, 1458 | 1576, 1426 |
B(O-H) | 1358 | 1352 | 1354 | 1354 | 1354 |
S(C-O-C) | 1202 | 1198 | 1198 | 1198 | 1194 |
S(C-OH) | 1096, 1054 | 1096, 1052 | 1122, 1054 | 1102, 1056 | 1138, 1074 |
The XRD pattern of the prepared GO shows (001) and (100) diffraction peaks at 2θ = 10.7 and 42.1° with d-spacing of 8.25 and 2.14 Å, respectively. This result agrees with that reported in the literature for GO [38, 39], confirming the successful preparation of this compound. The effect of oxidizing agents such as H2O2, K2S2O8, (NH4)2S2O8, and mCPBA on the XRD pattern of GO is shown in Figure 2. Moreover, Table 2 compares the variation of diffraction peak position (2θ), intensity, and d-spacing before and after the treatment of GO. It can be seen that the intensity of the diffraction peaks for GO after treatment by oxidizing agents decreases drastically. Also, a phase transition from more crystalline graphene oxide to more amorphous state is observed where GO is treated by oxidizing agents. It seems that oxidizing agents can create and enlarge the hole defects in graphene oxide. The amorphous carbon has a broad diffraction peak at about 2θ = 20° [40–42]. Therefore, the broad peaks observed at 26.6, 24.2, 28.2, and 30.6° for GO treated by H2O2, K2S2O8, (NH4)2S2O8, and mCPBA, respectively, confirm the amorphous nature of these compounds. The change from ordered GO to an amorphous phase in different conditions has been previously stated [43–45].
Compound | GO phase 2θ (intensity, d-spacing) | Amorphous phase 2θ (intensity) |
GO | 10.7 (1171.4, 8.25), 42.1 (395.2, 2.14) | 29.3 (43.0) |
GO treated by H2O2 | 11.0 (71.6, 8.01), 42.5 (75.1, 2.13) | 26.6 (191.3) |
GO treated by K2S2O8 | 42.8 (87.1, 2.11) | 24.2 (146.3) |
GO treated by (NH4)2S2O8 | 7.1 (79.7, 12.51), 42.4 (92.5, 2.13) | 28.2 (57.7) |
GO treated by mCPBA | 9.8 (395, 9.02), 42.5 (119.1, 2.12) | 30.6 (45.7) |
According to XRD patterns, the GO treated by mCPBA at room temperature has a more ordered structure (similar to GO) compared to the GO treated by other oxidizing agents at 80 °C, which is due to the formation of larger hole defects at higher temperatures. It has been reported that during the low-temperature and mid-temperature stages of graphene oxide preparation, the hole defects of graphene oxide would diminish significantly [35]. When the reaction temperature is over 50 °C, the graphene oxide becomes unstable and many holes in the graphene oxide due to excessive oxidation are formed [35].
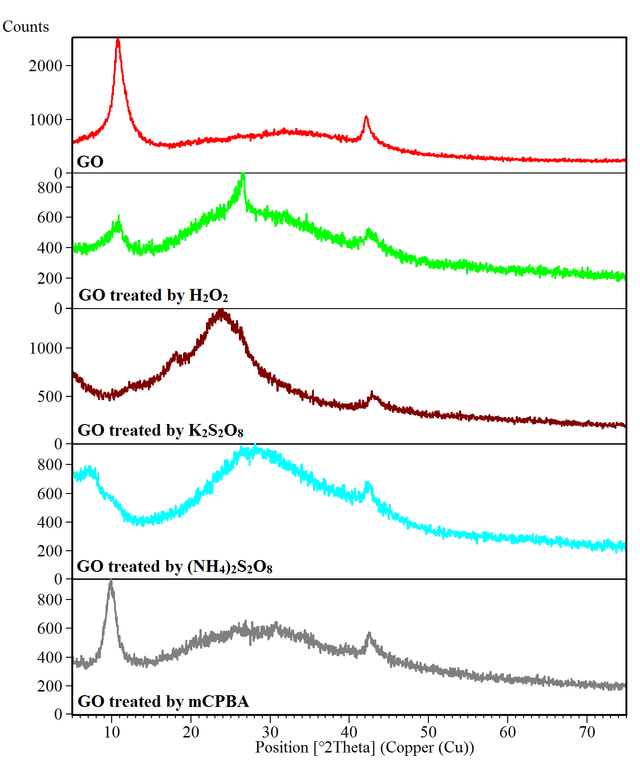
For the GO treated by mCPBA, the XRD peak of GO (001) from 10.7° shifts to a lower 2θ angle (9.8°) with increasing d-spacing from 8.25 to 9.02 Å. Similarly for GO treated by (NH4)2S2O8, this peak shifts to a lower 2θ angle (7.1°) with increasing d-spacing (12.51 Å). The highest deformation belongs to GO treated by K2S2O8 in which this peak shifts below 5°, and therefore is not observed in the recorded 2θ range. For GO treated by H2O2, the GO (001) peak shifts to a higher 2θ angle (11.0°) with decreasing d-spacing (8.01 Å). Both H2O2 and mCPBA add epoxy groups within the oxidized structure of GO. However since mCPBA is much larger than H2O2, the layer spacing expands. Persulfates moreover the epoxy groups, introduce carboxyl and hydroxyl functional groups to the surface, which is associated with increasing the interlayer spacing.
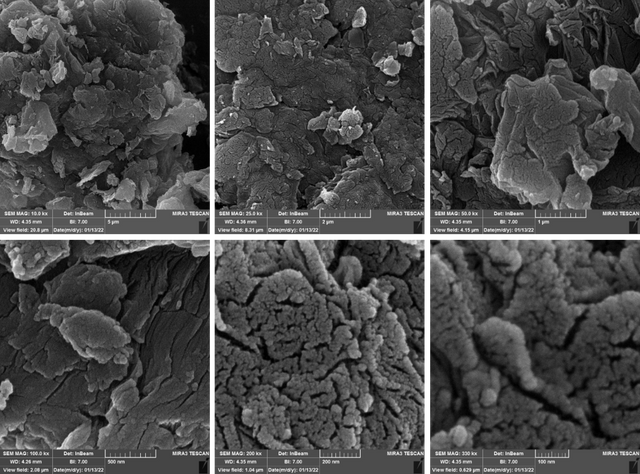
The FESEM micrographs of the GO prepared by the modified Hummers' method in this study are shown in Figure 3. It can be seen that the flakes of GO are tightly packed together in a sponge-like structure (due to the H-bonds between layers). The FE-SEM micrographs of GO treated by (NH4)2S2O8 and mCPBA are compared in Figs. 4 and 5, respectively. Significant changes due to the formation of hole defects in the morphology of the GO after treatment by oxidizing agents are observed via the FE-SEM micrographs.


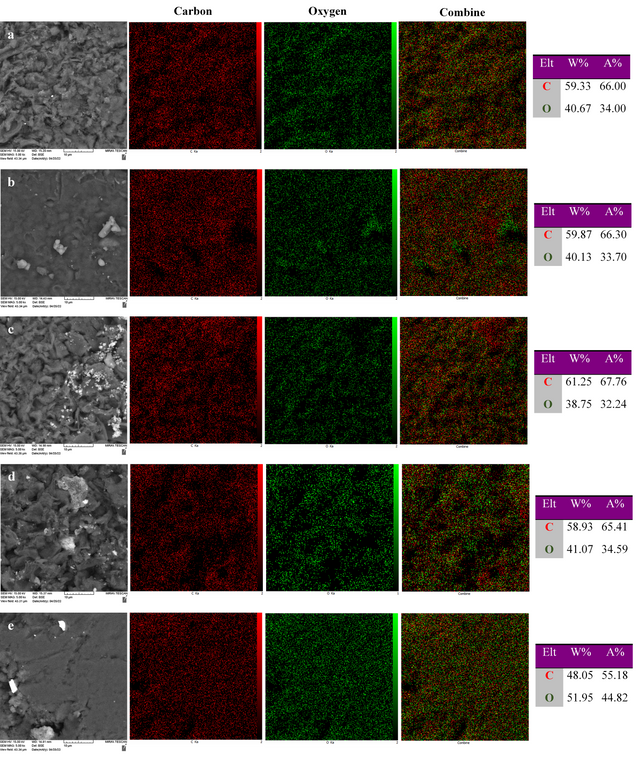
The base SEM images, elemental maps, and elemental compositions (weight (W) and atomic (A) percentages) according to EDX line scan analysis for GO and GO treated by oxidizing agents are indicated in Figure 6. The GO contains 66.00 A% (59.33 W%) C and 34.00 A% (40.67 W%) O. The high A% (or W%) of oxygen in GO confirms successful oxidation and the presence of oxygen-containing groups within the carbon layers. The carbon to oxygen atomic ratio is used to compare the relative levels of oxidation. This value decreases with increasing oxidation. The value of the C/O atomic ratio for GO is 1.94. Based on the EDX results, the highest % of O is found for GO treated by mCPBA (44.82 A%). The value of the C/O atomic ratio for this compound is 1.23. The GO treated by H2O2 contains 33.70 A% O. The value of the C/O atomic ratio for this compound is 1.97. The % of O for GO treated by K2S2O8 is 32.24 A%. The value of the C/O atomic ratio for this compound is 2.10. This value for GO treated by (NH4)2S2O8 is determined at 34.59 A%. The value of the C/O atomic ratio for this compound is 1.89. Therefore, GO treated by mCPBA shows a higher degree of oxidation than GO and GO treated by other oxidants.
Conclusions
GO was prepared by oxidation of graphite in a modified Hummers' method. The effects of peroxide and persulfate oxidizing agents (H2O2, K2S2O8, (NH4)2S2O8, and mCPBA) on the texture of GO were considered by FT-IR, XRD, FE-SEM, EDX, and elemental mapping techniques. According to FT-IR results, GO and GO treated by oxidizing agents contain a layer of carbon atoms that are decorated by oxygen-containing groups (i.e. hydroxyl, epoxy, carbonyl, and carboxyl). Based on XRD data, a phase transition from more crystalline graphene oxide to more amorphous state is observed where GO is treated by oxidizing agents. It seems that oxidizing agents can create and enlarge the hole defects in graphene oxide. The XRD pattern of GO treated by mCPBA at room temperature has a more ordered structure (similar to GO) compared to the GO treated by other oxidizing agents at 80 °C, which is due to the formation of larger hole defects at higher temperatures. Significant changes due to the formation of hole defects in the morphology of the GO after treatment by oxidizing agents are observed by the FE-SEM micrographs. The highest degree of oxidation is predicted for the GO treated by mCPBA.
Statements and Declarations
Authors' contributions
Y. Mahdavi: Investigation; M. Zamani: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Competing interests
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
No funding was received to support this work.
Acknowledgments
We would like to thank the research committee of Damghan University for supporting this work. Also, we thank Dr. Ali Reza Pourali and Maryam Korivand for their assistance in the synthesis of GO.
Authors’ Information
Mehdi Zamani—School of Chemistry, Damghan University, Damghan 36716-41167, Iran;
Yasaman Mahdavi—School of Chemistry, Damghan University, Damghan 36716-41167, Iran.



.jpg)
 - Copy copy.png)


